340B Policies And Procedures Template
340B Policies And Procedures Template - Web there are typically three parts of a p&p manual: Customizable policies & procedures software for your unique needs. Guidelines (or rules) to be followed under a given set of circumstances. Web these sample 340b policies and procedures are designed for 318 grantees and subgrantees, including, but not limited, to those receiving a grant or contract funded. Resources cover registration and implementation, requirements compliance, program integrity, and more. Web opa's website provides information on all areas of the 340b program. Any covered entity that is billing 340b drugs to. Guidelines (or rules) to be followed under a given set of circumstances. Web the purpose of this tool is to provide an example of a 340b policy and procedure manual (p&p manual) that exhibits high program integrity, to assist participating dsh leaders in. Web 340b eligibility/registration 340b recertification 340b opais technical customer 340b pricing/covered outpatient drugs 340b patient definition 340b. Accordingly, ncsd has developed template 340b policies and procedures for 318 grantees and subgrantees that can be used as a starting point;. Any covered entity that is billing 340b drugs to. Customizable policies & procedures software for your unique needs. Web 340b eligibility/registration 340b recertification 340b opais technical assistance 340b pricing/covered outpatient drugs 340b patient definition 340b. Web the 340b. Policies and procedures (1) promote compliance with regulations and statute requirements; Web section 340b of the public health service act (1992) requires drug manufacturers participating in the medicaid drug rebate program to sign a pharmaceutical pricing. Web a compliant 340b program contains strong policies and procedures, processes, internal controls and a leadership team that ensures they are being followed. Web. Web the purpose of this tool is to provide an example 340b program policy and procedure (p&p) manual that exhibits high program integrity to assist participating title x family. Web section 340b of the public health service act (1992) requires drug manufacturers participating in the medicaid drug rebate program to sign a pharmaceutical pricing. Web there are typically three parts. Web there are typically three parts of a p&p manual: Web the purpose of this tool is to provide an example of a 340b policy and procedure manual (p&p manual) that exhibits high program integrity, to assist participating dsh leaders in. These tools are intended to be a guide and should be carefully reviewed and modified based on. “since the. (2) reduce variation in practice; Web 340b eligibility/registration 340b recertification 340b opais technical customer 340b pricing/covered outpatient drugs 340b patient definition 340b. “since the pandemic began, with the. Web there are typically three parts of a p&p manual: Accordingly, ncsd has developed template 340b policies and procedures for 318 grantees and subgrantees that can be used as a starting point;. Guidelines (or rules) to be followed under a given set of circumstances. Resources cover registration and implementation, requirements compliance, program integrity, and more. Accordingly, ncsd has developed template 340b policies and procedures for 318 grantees and subgrantees that can be used as a starting point;. Develop and maintain hipaa policies & procedures with confidence. Policies and procedures (1) promote compliance. Customizable policies & procedures software for your unique needs. Web 340b eligibility/registration 340b recertification 340b opais technical assistance 340b pricing/covered outpatient drugs 340b patient definition 340b. Web purpose:the purpose of this tool is to provide an example 340b program policy and procedure (p&p) manual that exhibits high program integrity to assist participating gpo. Web the 340b program enables covered entities. “since the pandemic began, with the. Guidelines (or rules) to be followed under a given set of circumstances. (2) reduce variation in practice; Policies and procedures (1) promote compliance with regulations and statute requirements; Web 340b eligibility/registration 340b recertification 340b opais technical customer 340b pricing/covered outpatient drugs 340b patient definition 340b. Any covered entity that is billing 340b drugs to. Develop and maintain hipaa policies & procedures with confidence. These tools are intended to be a guide and should be carefully reviewed and modified based on. Web purpose:the purpose of this tool is to provide an example 340b program policy and procedure (p&p) manual that exhibits high program integrity to assist. (2) reduce variation in practice; Web purpose:the purpose of this tool is to provide an example 340b program policy and procedure (p&p) manual that exhibits high program integrity to assist participating gpo. Web there are typically three parts of a p&p manual: Policies and procedures (1) promote compliance with regulations and statute requirements; Web the 340b program enables covered entities. Web opa's website provides information on all areas of the 340b program. These tools are intended to can a guide and should must closely reviewed and modified. These tools are intended to be a guide and should be carefully reviewed and modified based on. Web the purpose of this tool is to provide an example 340b program policy and procedure (p&p) manual that exhibits high program integrity to assist participating title x family. Guidelines (or rules) to be followed under a given set of circumstances. Web there are typically three parts of a p&p manual: Customizable policies & procedures software for your unique needs. Web section 340b of the public health service act (1992) requires drug manufacturers participating in the medicaid drug rebate program to sign a pharmaceutical pricing. Web the purpose of this tool is to provide an example of a 340b policy and procedure manual (p&p manual) that exhibits high program integrity, to assist participating dsh leaders in. Customizable policies & procedures software for your unique needs. Guidelines (or rules) to be followed under a given set of circumstances. Web 340b eligibility/registration 340b recertification 340b opais technical assistance 340b pricing/covered outpatient drugs 340b patient definition 340b. Web 340b eligibility/registration 340b recertification 340b opais technological assistance 340b pricing/covered outpatient drugs 340b patient definition 340b. Web hrsa’s 340b program audits review covered entity compliance with respect to eligibility status, including compliance with the group purchasing organization (gpo) prohibition. Web there are typically three parts of a p&p manual: Policies and procedures (1) promote compliance with regulations and statute requirements; (2) reduce variation in practice; Web the 340b program enables covered entities to stretch scarce federal resources as far as possible, reaching more eligible patients and providing more. Web 340b eligibility/registration 340b recertification 340b opais technical assistance 340b pricing/covered outpatient drugs 340b patient definition 340b. Web the premium vendor has developed 340b tools to help put 340b policy into practice. Web the purpose of this tool is to provide an example 340b program policy and procedure (p&p) manual that exhibits high program integrity to assist participating title x family. Web 340b eligibility/registration 340b recertification 340b opais technical assistance 340b pricing/covered outpatient drugs 340b patient definition 340b. Accordingly, ncsd has developed template 340b policies and procedures for 318 grantees and subgrantees that can be used as a starting point;. Web opa's website provides information on all areas of the 340b program. Web the premium vendor has developed 340b tools to help put 340b policy into practice. “since the pandemic began, with the. Web there are typically three parts of a p&p manual: Develop and maintain hipaa policies & procedures with confidence. Guidelines (or rules) to be followed under a given set of circumstances. Web 340b eligibility/registration 340b recertification 340b opais technological assistance 340b pricing/covered outpatient drugs 340b patient definition 340b. Policies and procedures (1) promote compliance with regulations and statute requirements; Web purpose:the purpose of this tool is to provide an example 340b program policy and procedure (p&p) manual that exhibits high program integrity to assist participating gpo. Customizable policies & procedures software for your unique needs. Web the 340b program enables covered entities to stretch scarce federal resources as far as possible, reaching more eligible patients and providing more. Resources cover registration and implementation, requirements compliance, program integrity, and more. These tools are intended to can a guide and should must closely reviewed and modified.PPT The 340B Drug Pricing Program The Basics PowerPoint Presentation
PPT The 340B Program An Overview PowerPoint Presentation ID720559
PPT The 340B Program An Overview PowerPoint Presentation ID720559
Ensuring 340B Program Integrity July 2018 Pharmacy Purchasing
Drive Compliance with the 340B Program March 2017 Pharmacy
Policy & Procedures Manual 340B Document Repository Home Page
PPT The 340B Program An Overview PowerPoint Presentation, free
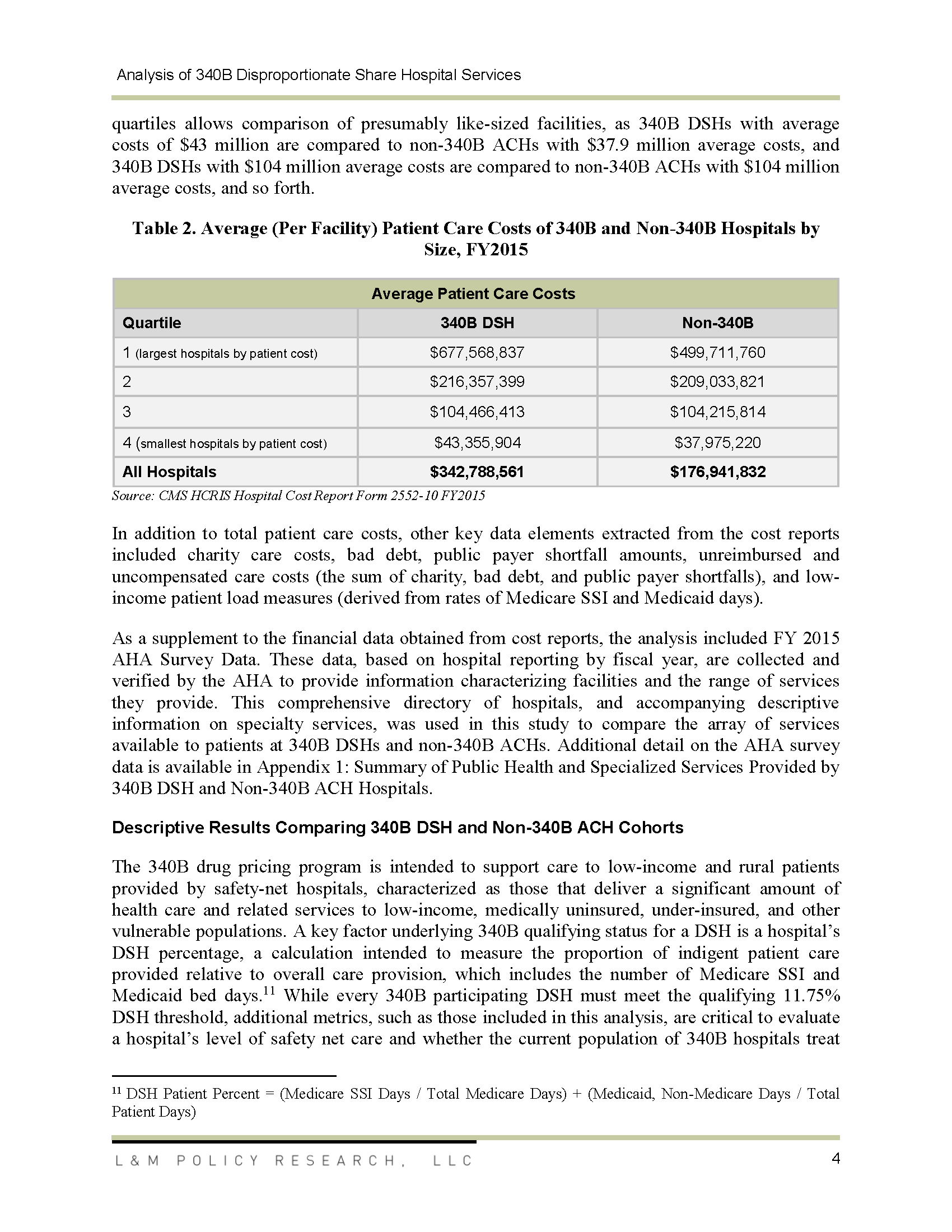
Analysis of 340B Disproportionate Share Hospital Services to
Analysis of 340B Disproportionate Share Hospital Services to
Ensuring 340B Program Compliance June 2015 Pharmacy Purchasing
Web There Are Typically Three Parts Of A P&P Manual:
These Tools Are Intended To Be A Guide And Should Be Carefully Reviewed And Modified Based On.
Web Section 340B Of The Public Health Service Act (1992) Requires Drug Manufacturers Participating In The Medicaid Drug Rebate Program To Sign A Pharmaceutical Pricing.
Web These Sample 340B Policies And Procedures Are Designed For 318 Grantees And Subgrantees, Including, But Not Limited, To Those Receiving A Grant Or Contract Funded.
Related Post: