Clinical Study Report Template Word
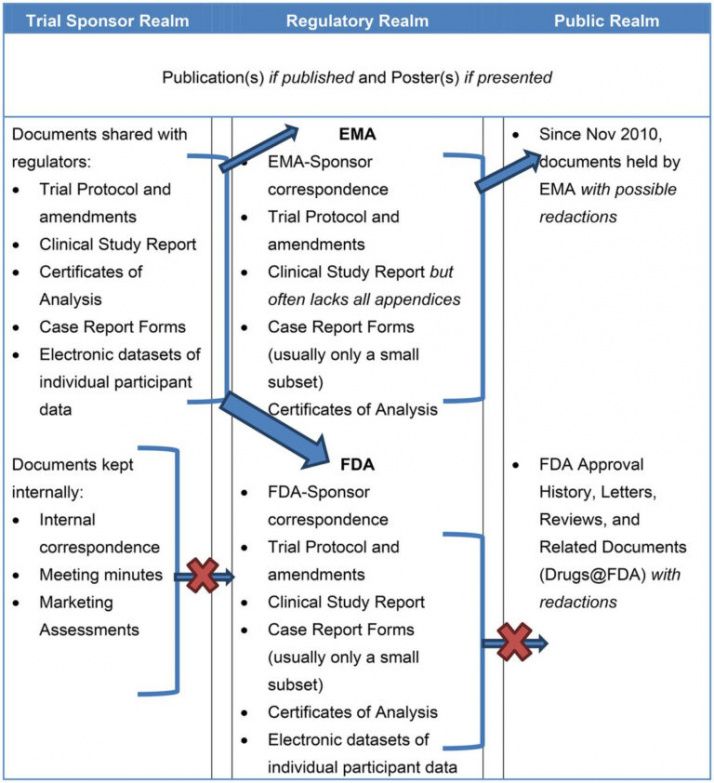
Clinical Study Report Template Word - Center for drug evaluation and research the objective of this guideline is to facilitate the compilation of a single core clinical study report. Using our solution filling out clinical study report. Instead, it is a scientific. Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here. Avail yourself of an example to create a. Web clinical study report [study title] [study number] [dd month yyyy] confidential signature pages for clinical study report i have read this report and confirm that to the. Web the full clinical study report (csr) encompasses all aspects and details of the research you’ve conducted. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Web the toolbox contains resources such as nih and nimh policy and guidance documents, templates, sample forms, links to additional resources, and other materials to assist. Monitoring plans comprehensive monitoring plan template; This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web the toolbox contains resources such as nih and nimh policy and guidance documents, templates, sample forms, links to additional resources, and other materials to assist. The forms serve only as. Web clinical study report [study title] [study number] [dd month yyyy] confidential signature pages for clinical study report i have read this report and confirm that to the. The forms serve only as templates, and must be. Try scribd free for 30 days. Budget monitoring tool with example data. Web write a medical report for a health examination result or. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web write a medical report for a health examination result or hospital treatment of a patient with our free download sample templates. Web clinical case study presentation template. Using our solution filling out clinical study report. Try. Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here. It is not a sales or marketing tool; Instead, it is a scientific. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Avail yourself of an example to. Web write a medical report for a health examination result or hospital treatment of a patient with our free download sample templates. Using our solution filling out clinical study report. Instead, it is a scientific. It is not a sales or marketing tool; Monitoring plans comprehensive monitoring plan template; Monitoring plans comprehensive monitoring plan template; Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Ad access millions of ebooks, audiobooks, podcasts, and more. Web clinical study. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Web clinical study report template. The forms serve only as templates, and must be. Instead, it is a scientific. Web inventory list for study storage documents; Web write a medical report for a health examination result or hospital treatment of a patient with our free download sample templates. Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here. Experience all the key benefits of submitting and completing forms on the internet. Web the clinical study report described. The forms serve only as templates, and must be. Csr + csp (10% off) + csps (free) click for price. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web clinical case study presentation template. Plus, resources to support their use,. Cdi software from 3m uses ai and integrated workflows to achieve accurate documentation. It is not a sales or marketing tool; Monitoring plans comprehensive monitoring plan template; Web write a medical report for a health examination result or hospital treatment of a patient with our free download sample templates. Web follow the simple instructions below: Experience all the key benefits of submitting and completing forms on the internet. Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here. Web the toolbox contains resources such as nih and nimh policy and guidance documents, templates, sample forms, links to additional resources, and other materials to assist. Web clinical study report (csr) template. With scribd, you can take your ebooks and audibooks anywhere, even offline. Web clinical study report template. Ad 3m cdi solutions use shared workflows to improve documentation and quality scores. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. Web the full clinical study report (csr) encompasses all aspects and details of the research you’ve conducted. Web clinical researcher—september 2020 (volume 34, issue 8) peer reviewed sheryl stewart, mcr, ccrp the philosophy of good clinical custom. Avail yourself of an example to create a. Downloadable ms word template (.dotx) file type that is automatically converted to a standard ms word document (. Web inventory list for study storage documents; The niams has guidelines and templates to help investigators develop a study mop. The forms serve only as templates, and must be. A clinical case study is a report where medical practitioners share a. Budget monitoring tool with example data. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Web follow the simple instructions below: Ad 3m cdi solutions use shared workflows to improve documentation and quality scores. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web clinical study report [study title] [study number] [dd month yyyy] confidential signature pages for clinical study report i have read this report and confirm that to the. Experience all the key benefits of submitting and completing forms on the internet. Web follow the simple instructions below: Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. With scribd, you can take your ebooks and audibooks anywhere, even offline. Csr + csp (10% off) + csps (free) click for price. Web clinical study report (csr) template. Budget monitoring tool with example data. Using our solution filling out clinical study report. Downloadable ms word template (.dotx) file type that is automatically converted to a standard ms word document (. Web clinical study report template. Web the toolbox contains resources such as nih and nimh policy and guidance documents, templates, sample forms, links to additional resources, and other materials to assist. Plus, resources to support their use,. Web inventory list for study storage documents;Pin on Numbers
Clinical Trial Report Template (2) TEMPLATES EXAMPLE TEMPLATES
Clinical Trial Report Template (1) TEMPLATES EXAMPLE TEMPLATES
Pin on Report Template
Trial Report Template
Clinical Study Report Template PDF Sample Stableshvf
12+ Research Report Templates Free Apple Pages, Google Docs, Word
Pin on Report Template
Monitoring Report Template Clinical Trials
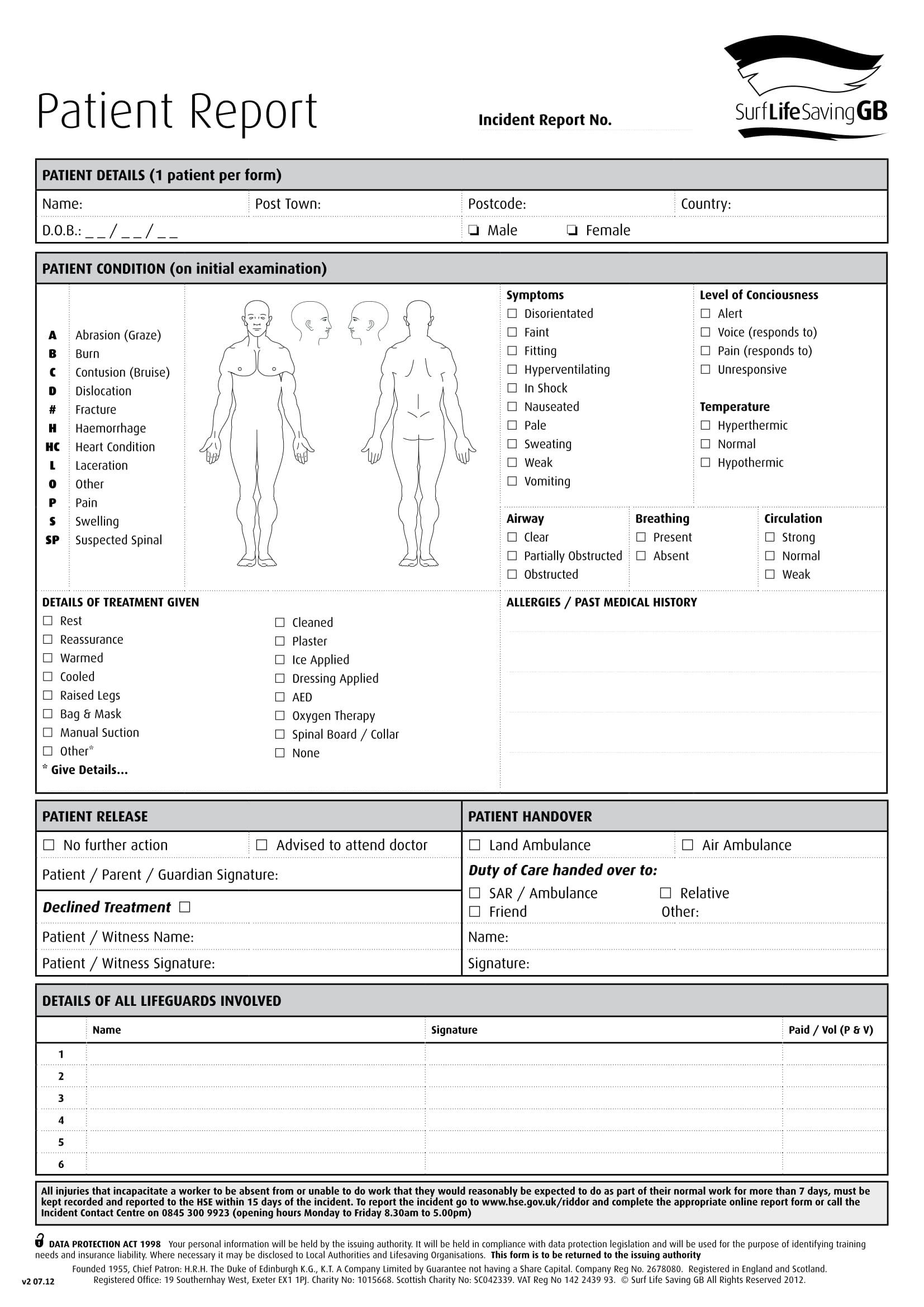
Patient Report Form Template Download Best Template Ideas
Instead, It Is A Scientific.
A Clinical Case Study Is A Report Where Medical Practitioners Share A.
Center For Drug Evaluation And Research The Objective Of This Guideline Is To Facilitate The Compilation Of A Single Core Clinical Study Report.
Web This Clinical Study Report (Csr) Template Is Specifically Designed To Be Used In Conjunction With The Clinical Study Protocol (Csp) Template.
Related Post: