Corrective And Preventive Action Plan Template
Corrective And Preventive Action Plan Template - Web corrective and preventive action (capa) plans. Web corrective action and preventive action (capa) plan template. Web the purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality. Ad flexible & easy to use. Sop corrective and preventive action (capa) dr. Web capa, or corrective action and preventive action, can provide a structure for finding the root cause of problems, solving those problems, documenting the. Ad prevent failures & reduce costs with a preventive maintenance software program. • distinguish between a corrective action and preventative action. This template is designed to. Ad read quick faqs and download compliance checklist. Web a corrective and preventive action report outlines the work or process improvements required to eliminate a particular defect/set of defects and reduce the likelihood of non. Oliver eidel template download this is a free template, provided by openregulatory. Take immediate corrective actions if you become aware of a deviation or unexpected event that endangers the rights, welfare, or safety. Web a corrective and preventive action report outlines the work or process improvements required to eliminate a particular defect/set of defects and reduce the likelihood of non. Download corrective and preventative action plan form template_2019.11.13. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Oliver eidel template download. This template is designed to. Web • demonstrate writing and developing effective corrective and preventative action plans. With the help of this plan, you. Web a corrective action plan is used by the companies and organizations to deal with and prevent any undesirable behaviors and situations at work. Web learning objectives know the purpose of corrective and preventive action have. Web capa, or corrective action and preventive action, can provide a structure for finding the root cause of problems, solving those problems, documenting the. With the help of this plan, you. Ad prevent failures & reduce costs with a preventive maintenance software program. The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and. Ad. Oliver eidel template download this is a free template, provided by openregulatory. Ad flexible & easy to use. With the help of this plan, you. A corrective action plan is comprised of different activities that must be planned and implemented in an organized. Web corrective action and preventive action (capa) plan template. Preventive maintenance software streamlines work, tracks inventory, and manages assets. Ad read quick faqs and download compliance checklist. Web corrective action and preventive action (capa) plan template. Ad 1) create a corrective action plan for your business. With the help of this plan, you. • distinguish between a corrective action and preventative action. With the help of this plan, you. Web the purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality. Ad read quick faqs and download compliance checklist. Web • demonstrate writing and developing effective corrective and preventative action plans. Oliver eidel template download this is a free template, provided by openregulatory. This template is designed to. Ad read quick faqs and download compliance checklist. Ad read quick faqs and download compliance checklist. Web corrective and preventive actions (capa) plans. With the help of this plan, you. Sop corrective and preventive action (capa) dr. A corrective action plan is comprised of different activities that must be planned and implemented in an organized. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. • distinguish between a corrective action and preventative action. Sop corrective and preventive action (capa) dr. Preventive maintenance software streamlines work, tracks inventory, and manages assets. Web learning objectives know the purpose of corrective and preventive action have the ability to distinguish between each defined term understand the requirements in 21 cfr. Web corrective action and preventive action (capa) plan template. Web corrective and preventive actions (capa) plans. The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and. Web corrective and preventive actions (capa) plans. Web a corrective action plan is used by the companies and organizations to deal with and prevent any undesirable behaviors and situations at work. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Preventive maintenance software streamlines work, tracks inventory, and manages assets. Web • demonstrate writing and developing effective corrective and preventative action plans. With the help of this plan, you. Take immediate corrective actions if you become aware of a deviation or unexpected event that endangers the rights, welfare, or safety of participants and others,. Web a corrective and preventive action report outlines the work or process improvements required to eliminate a particular defect/set of defects and reduce the likelihood of non. Ad flexible & easy to use. This template is designed to. Sop corrective and preventive action (capa) dr. Ad read quick faqs and download compliance checklist. Guiding clinical research professionals in improving weaknesses, deficiencies, or in rectifying deviation patterns and areas of. Take note that utilizing a. A corrective action plan is comprised of different activities that must be planned and implemented in an organized. Download corrective and preventative action plan form template_2019.11.13. Ad read quick faqs and download compliance checklist. Web learning objectives know the purpose of corrective and preventive action have the ability to distinguish between each defined term understand the requirements in 21 cfr. Web the purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality. Ad 1) create a corrective action plan for your business. Sop corrective and preventive action (capa) dr. Web the iu hrpp quality improvement office (qio) has developed a capa plan template to assist study teams with this activity. Web corrective action and preventive action (capa) plan template. With the help of this plan, you. Take immediate corrective actions if you become aware of a deviation or unexpected event that endangers the rights, welfare, or safety of participants and others,. Web capa, or corrective action and preventive action, can provide a structure for finding the root cause of problems, solving those problems, documenting the. Web a corrective and preventive action report outlines the work or process improvements required to eliminate a particular defect/set of defects and reduce the likelihood of non. Web learning objectives know the purpose of corrective and preventive action have the ability to distinguish between each defined term understand the requirements in 21 cfr. The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and. Web the purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality. Download corrective and preventative action plan form template_2019.11.13. Ad flexible & easy to use. Web • demonstrate writing and developing effective corrective and preventative action plans. • distinguish between a corrective action and preventative action. Ad read quick faqs and download compliance checklist.22+ Corrective Action Plan Templates Google Docs MS Word Apple
Sample, Example & Format Templates 11+ Safety Corrective Action Plan
12+ Corrective Action Report Examples Pdf in 2020 Report template
Corrective Action Plan Template Inspirational Corrective Action Plan
FREE 7+ Sample Preventive Action Forms in MS Word PDF Excel
FREE 7+ Sample Preventive Action Forms in MS Word PDF Excel
Pin on Business Plan Template for Startups
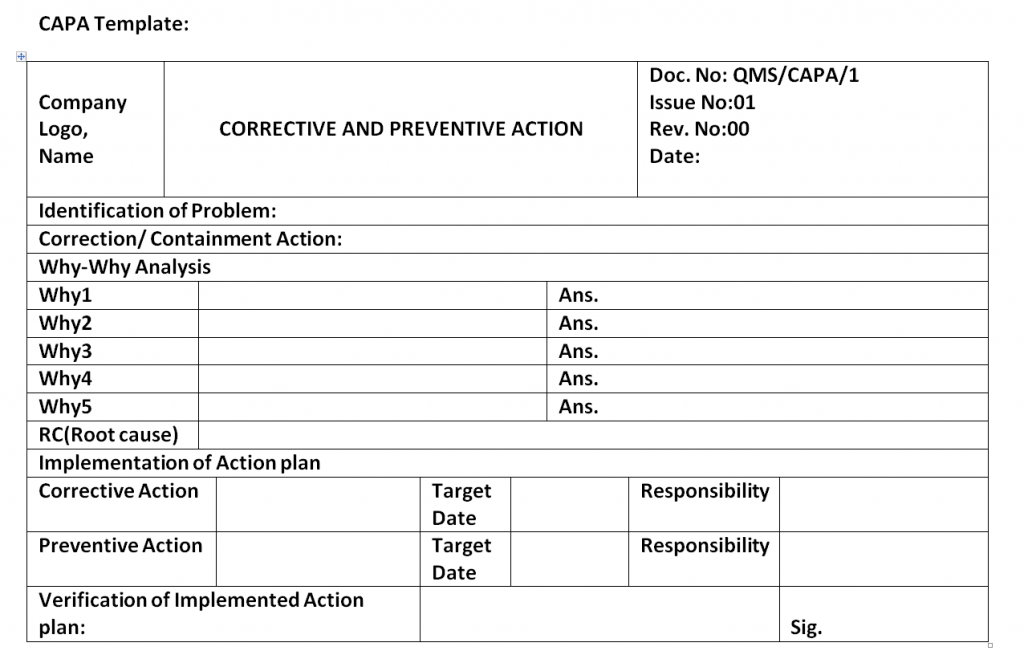
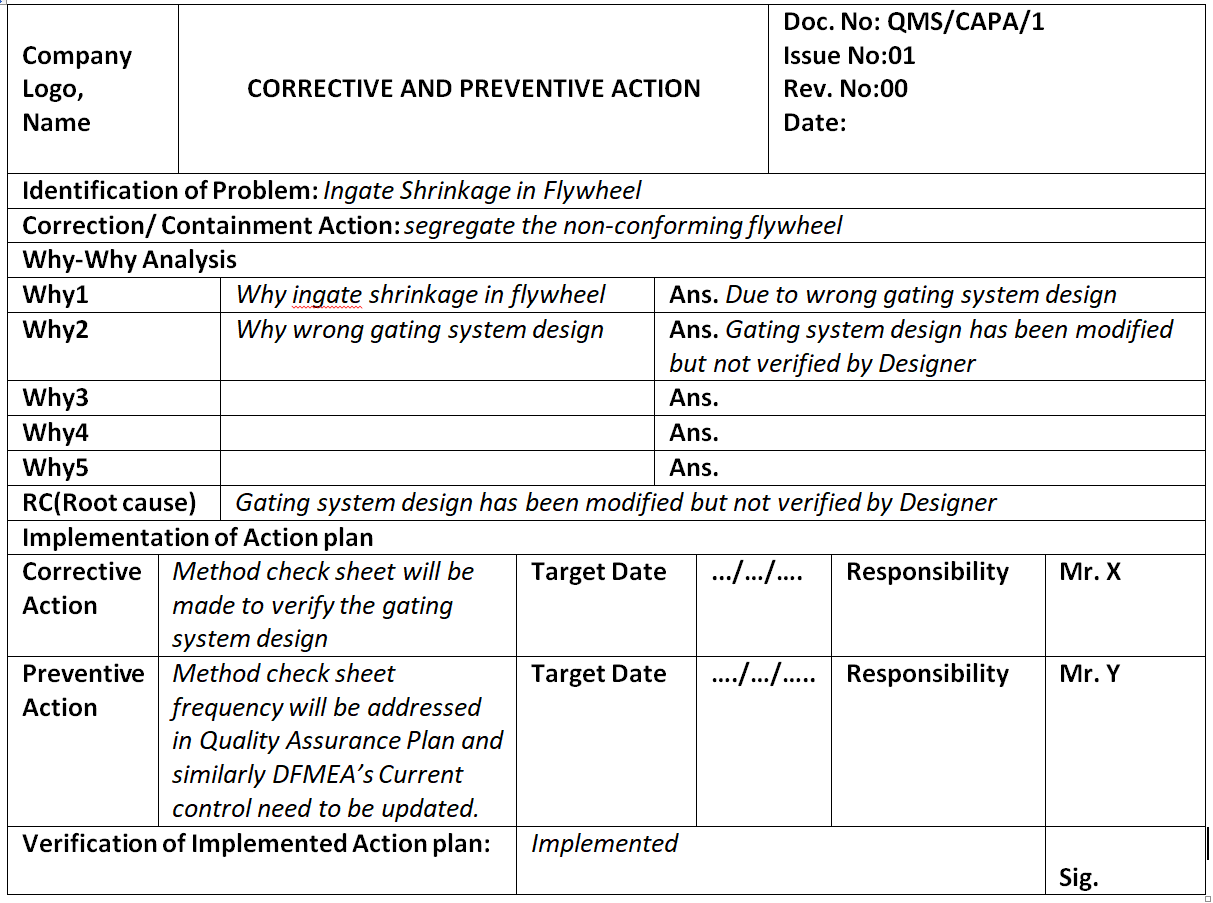
Corrective and Preventive Action Format CAPA with Example Download
Corrective and Preventive Action Format CAPA with Example
FREE 7+ Sample Preventive Action Forms in MS Word PDF Excel
Web Corrective And Preventive Action (Capa) Plans.
Ad Read Quick Faqs And Download Compliance Checklist.
Web A Corrective Action Plan Is Used By The Companies And Organizations To Deal With And Prevent Any Undesirable Behaviors And Situations At Work.
Guiding Clinical Research Professionals In Improving Weaknesses, Deficiencies, Or In Rectifying Deviation Patterns And Areas Of.
Related Post: