Device History Record Template
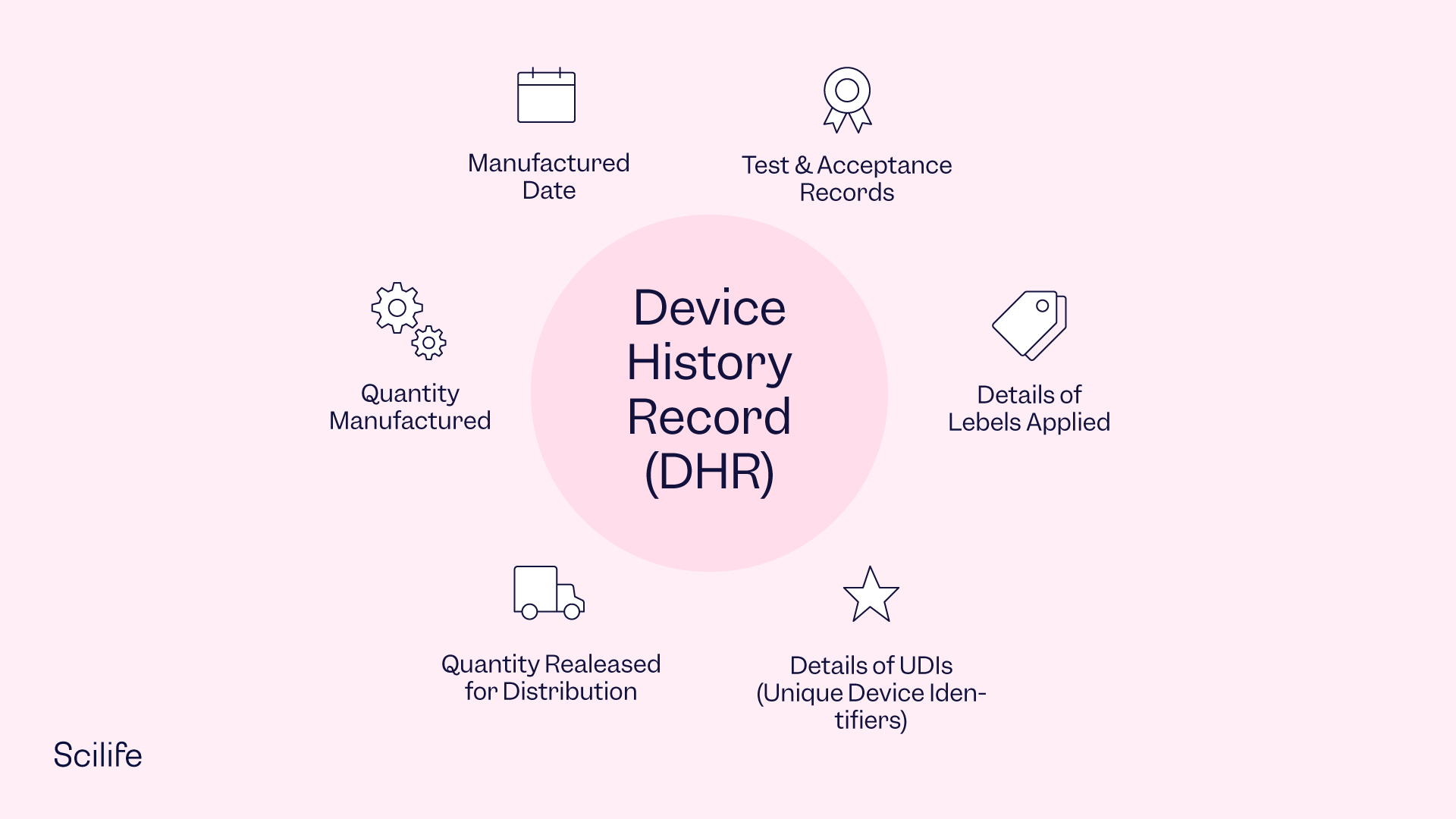
Device History Record Template - Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device. The specific contents of the device history record are reported within 21 cfr 820.184. Web fyi, there is no such thing as a dhr template. Web the device history record (dhr) demonstrates that all batch, lot, or manufacturing unit in a pharmaceutical device was manufactured according to the specifications in the device. Device history record dhfs for software in medical devices. 21 cfr 820.3 (i) provides the following definition: Describe requirements and intent for document controls,. The essential components of a dhf design history file vs. (d) the acceptance records which demonstrate the device is. The free family history records is a template that helps you organize the important information. Device account records (dhrs) are ampere crucial portion of the medizintechnik device quality management system. Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device. The specific contents of the device history record are reported within 21 cfr 820.184. Web a device history record. Web a device history record (dhr) refers to a compilation of records containing the production history of a finished device and is defined under subpart m 21 cfr part 820 (section. The history and information related to how you made the device, in accordance. This appendix covers the following. Describe requirements and intent for document controls,. The specific contents of. Device history record (dhr) means a compilation of records containing the production. The device history record (dhr) is outlined in the us fda quality system requirements, part 820, section 184. Web what is a design history file? The specific contents of the device history record are reported within 21 cfr 820.184. Web a device history record (dhr) refers to a. (c) the quantity released for distribution; The device history record (dhr) is outlined in the us fda quality system requirements, part 820, section 184. The specific contents of the device history record are reported within 21 cfr 820.184. (a) the dates of manufacture; Web the device history record (dhr) demonstrates that all batch, lot, or manufacturing unit in a pharmaceutical. Web fyi, there is no such thing as a dhr template. This appendix covers the following. Device account records (dhrs) are ampere crucial portion of the medizintechnik device quality management system. Web mdf record book template. Specifically, the dhr shall include: Web (a) the dates of manufacture; This appendix covers the following. The specific contents of the device history record are reported within 21 cfr 820.184. Web the device history record (dhr) demonstrates that all batch, lot, or manufacturing unit in a pharmaceutical device was manufactured according to the specifications in the device. The device history record is literally the history. Web what is a device history record (dhr)? [definition and components] read below if you would like more information regarding device history records and its. The history and information related to how you made the device, in accordance. Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the. Web it’s also helpful to your children and their children, further down the road of life. Device account records (dhrs) are ampere crucial portion of the medizintechnik device quality management system. It's the collection of documents and records of the fabrication,. Identify key definitions related to documents and records 2. Web the fully documentation about the manufacturing or tracking of. Web the “device history record”. The history and information related to how you made the device, in accordance. The essential components of a dhf design history file vs. Web the fully documentation about the manufacturing or tracking of every medical device that your company sold is contained in a device history record (dhr). The device history record (dhr) is outlined. This appendix covers the following. Web (a) the dates of manufacture; The essential components of a dhf design history file vs. Web for starters, you’ll need a digital master template and device history record, allowing you to review, approve and complete a master template to create edhr. Web the “device history record”. Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device. The free family history records is a template that helps you organize the important information. Web (a) the dates of manufacture; 21 cfr 820.3 (i) provides the following definition: It's the collection of documents and records of the fabrication,. Web what is a design history file? Simply because, it is unique to your device and system. (d) the acceptance records which demonstrate the device is. (d) the acceptance records which demonstrate the device is. [definition and components] read below if you would like more information regarding device history records and its. Web mdf record book template. Device account records (dhrs) are ampere crucial portion of the medizintechnik device quality management system. Web the “device history record”. (c) the quantity released for distribution; The specific contents of the device history record are reported within 21 cfr 820.184. Identify key definitions related to documents and records 2. The essential components of a dhf design history file vs. The device history record (dhr) is outlined in the us fda quality system requirements, part 820, section 184. Specifically, the dhr shall include: Web a device history record (dhr) refers to a compilation of records containing the production history of a finished device and is defined under subpart m 21 cfr part 820 (section. It's the collection of documents and records of the fabrication,. Web what is a device history record (dhr)? (c) the quantity released for distribution; (a) the dates of manufacture; By using medical device qms. Device history record dhfs for software in medical devices. The free family history records is a template that helps you organize the important information. The specific contents of the device history record are reported within 21 cfr 820.184. Web for starters, you’ll need a digital master template and device history record, allowing you to review, approve and complete a master template to create edhr. Device account records (dhrs) are ampere crucial portion of the medizintechnik device quality management system. Web (a) the dates of manufacture; Web device history record shall be defined as the compilation of records containing the complete production / maintenance history of a finished product and showing latest. (d) the acceptance records which demonstrate the device is. (d) the acceptance records which demonstrate the device is. Web mdf record book template. Web in medical device and diagnostic manufacturing, companies must keep a complete and accurate record of each product they produce in the form of a device.What is Device History Record (DHR)? Complete definition Scilife
Federal Register Unique Device Identification System
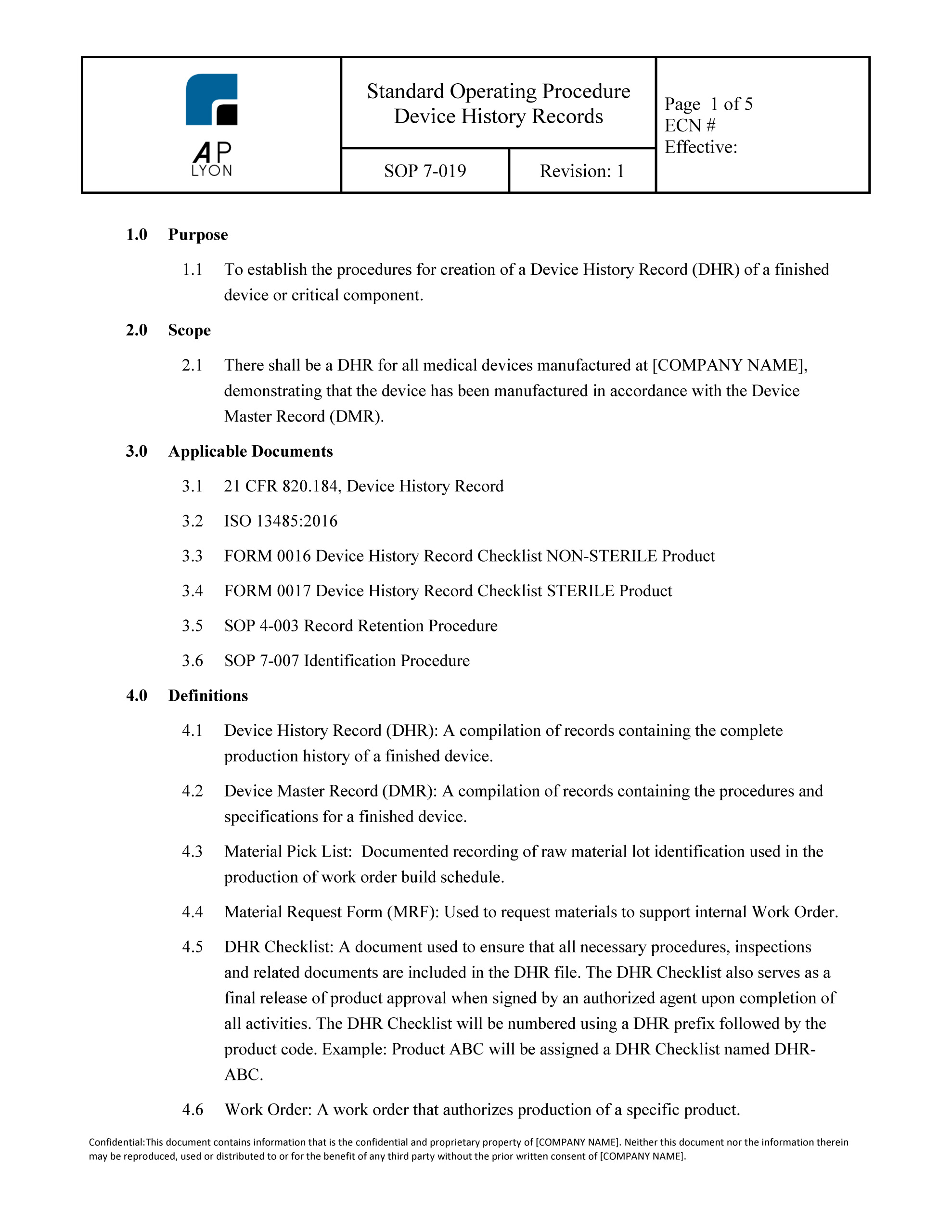
Device History Record Procedure
PPT Design documentation PowerPoint Presentation ID1625484
Why You Should Keep a Record of Device Serial Numbers Churches of
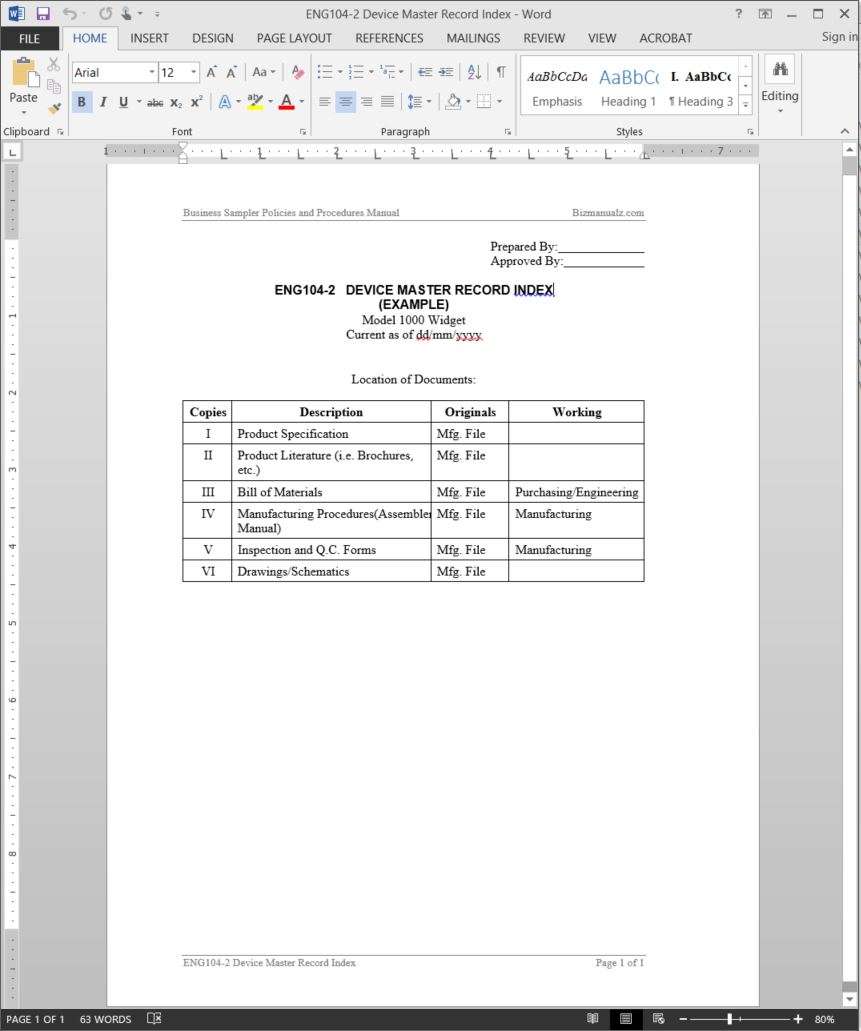
Device Master Record Index Template
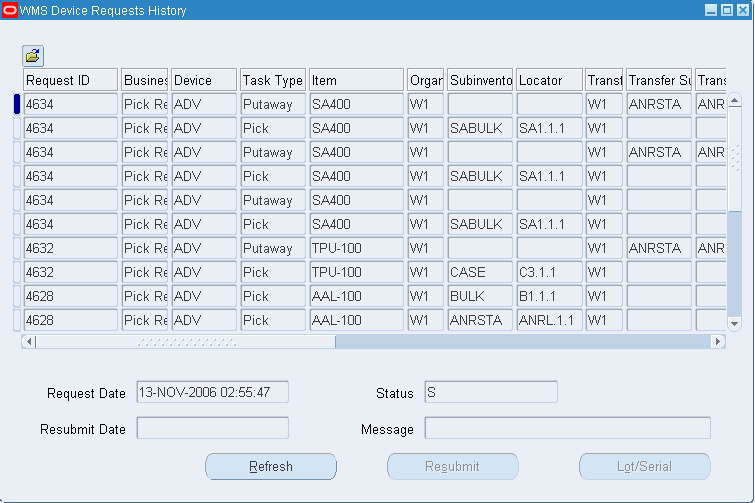
Oracle Warehouse Management User's Guide
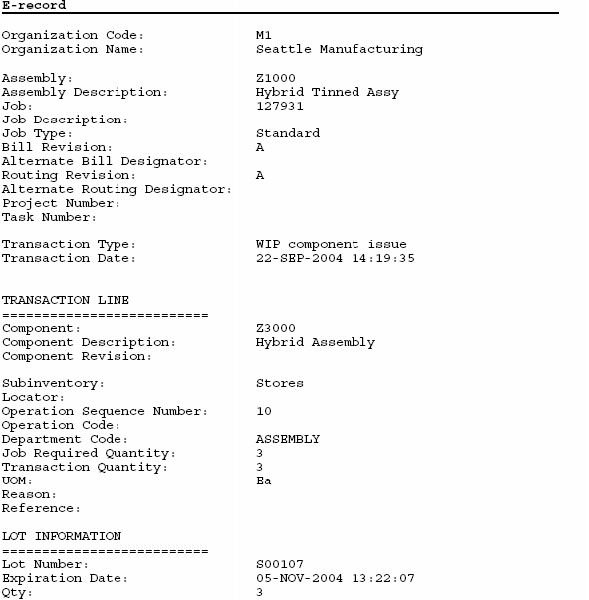
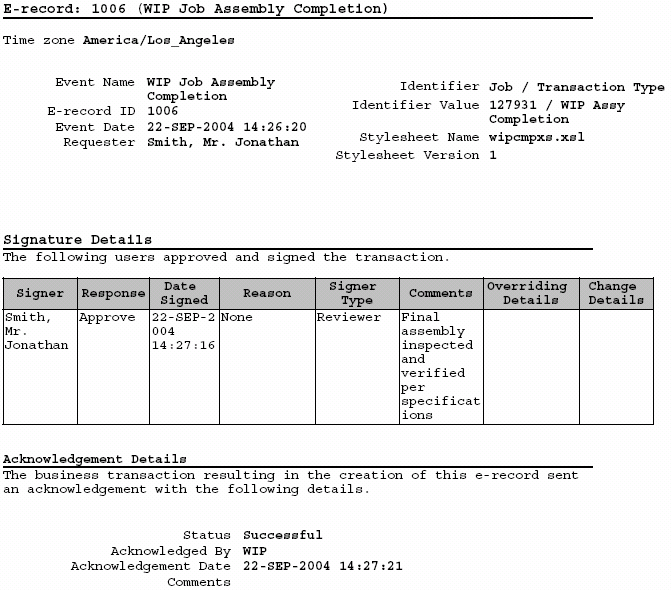
Oracle Manufacturing Implementing Oracle ERecords in Discrete
Oracle Manufacturing Implementing Oracle ERecords in Discrete
Device Master Records & Design History Files
The History And Information Related To How You Made The Device, In Accordance.
Web A Device History Record (Dhr) Contains All The Documents That Are Related To The Manufacturing And Tracking Of A Medical Device.
Web What Is A Design History File?
Web Fyi, There Is No Such Thing As A Dhr Template.
Related Post: