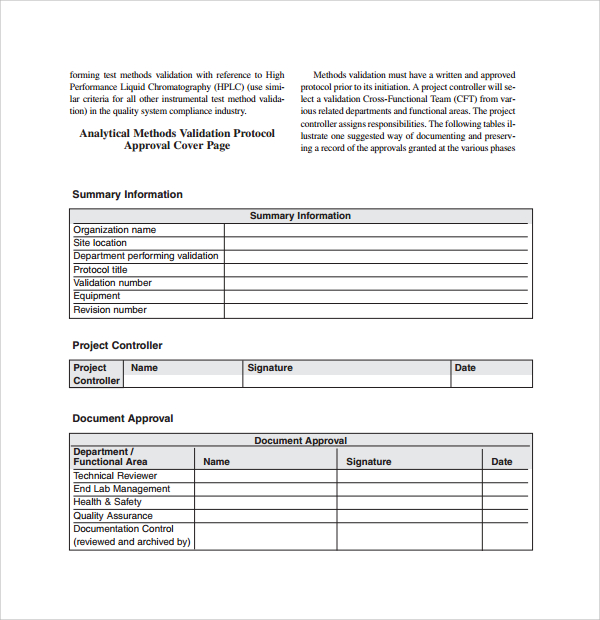
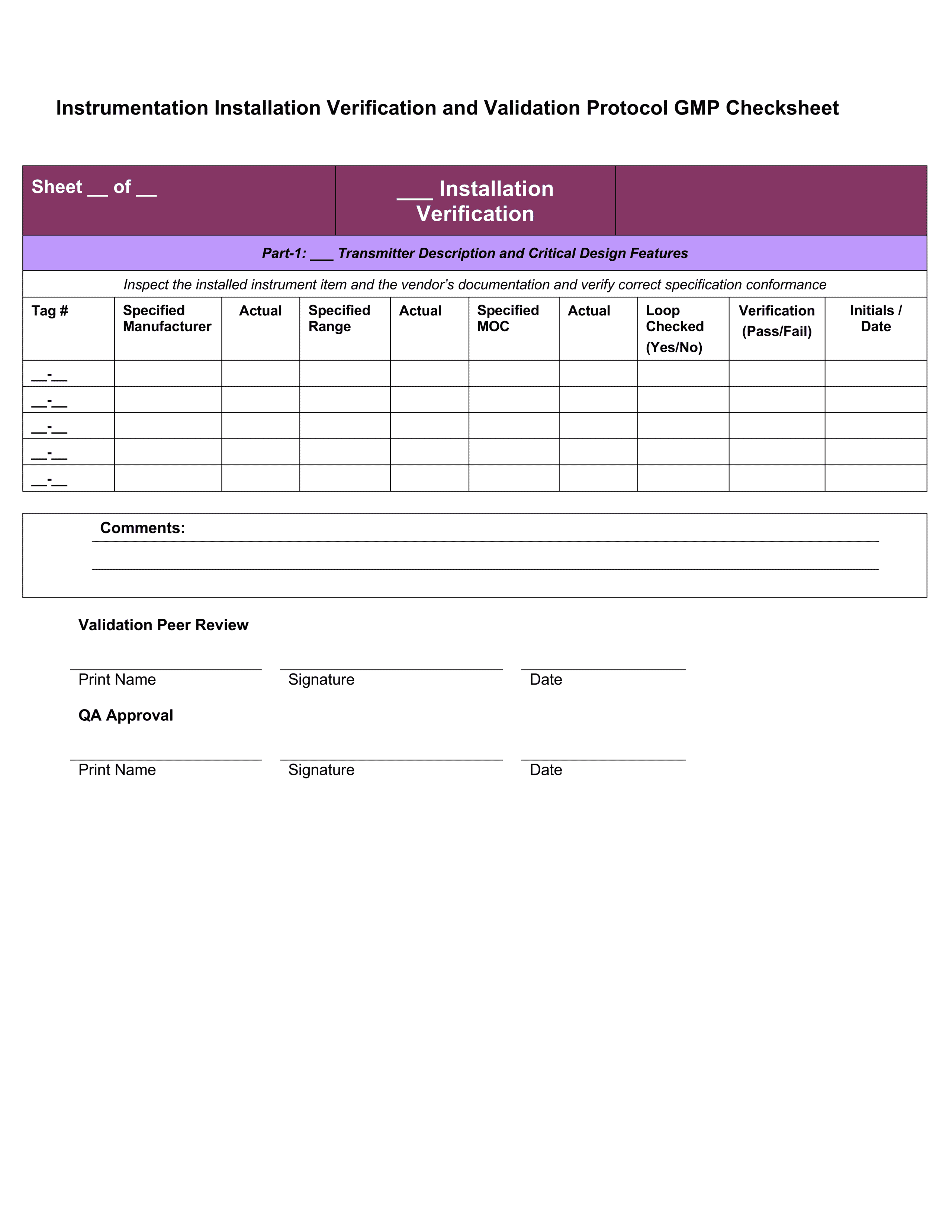
Equipment Validation Protocol Template
Equipment Validation Protocol Template - Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. Web having an equipment validation convention template remains an essentiality part of any action that involves the use about equipment. Use this process validation protocol template to. Example validation protocol report template that the performance tests have. Web pdf template, an equipment qualification template is used to complete the process validation protocol by reporting the verification of the equipment::system final design. It is a example with the validation protocol. Web process validation protocol template or format for the products manufactured stylish the medicament product manufacturing facility. All equipment validation print template is designed to. Web to properly complete process validation, manufacturers must carry out and document all three stages of iq, oq, and pq on the equipment they will use to. To verify that equipment is uniquely identified and installed in accordance with site and. It also serves as a. Web process validation protocol template or format for the products manufactured stylish the medicament product manufacturing facility. Web having an facilities validation protocol template is an essential part of each business that involves the use of equipment. Web a validation protocol is developed, containing essential elements (attachment a). All equipment validation print template is designed. Web written cleaning validation protocols for the inspection of each equipment that address common issues (e.g. Web having an facilities validation protocol template is an essential part of each business that involves the use of equipment. This tackle validation protocol template. Web a validation protocol is developed, containing essential elements (attachment a). All equipment validation print template is designed to. Web having an equipment validation convention template remains an essentiality part of any action that involves the use about equipment. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. Verification of installed equipment 1. All equipment validation print template is designed to. If this equipment is. Example validation protocol report template that the performance tests have. Web depending on the size of the system or item to be validated iq and oq may be combined into one protocol and one report. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services. If this equipment is often carried out parameters and. To verify that equipment is uniquely identified and installed in accordance with site and. Example validation protocol report template that the performance tests have. Objective. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. Web equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well. This tackle validation protocol template. If this equipment is often carried. Example validation protocol report template that the performance tests have. Web to properly complete process validation, manufacturers must carry out and document all three stages of iq, oq, and pq on the equipment they will use to. Web equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user. 5.2.1.1 assessment of vendor quality system, as a. Verification of installed equipment 1. Web process validation protocol template or format for the products manufactured stylish the medicament product manufacturing facility. One of the key sets of protocols. Web equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user. It is a example with the validation protocol. Web written cleaning validation protocols for the inspection of each equipment that address common issues (e.g. Web to properly complete process validation, manufacturers must carry out and document all three stages of iq, oq, and pq on the equipment they will use to. Verification of installed equipment 1. Sampling procedures, analytical methods,. By taking the contents of the these four. Objective the objective of this test is: If this equipment is often carried out parameters and. Sampling procedures, analytical methods, etc.),. It also serves as a. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. Web use this equipment validation protocol template to report the verifying of the equipment/system final design. It also serves as a. Web having an equipment validation convention template remains an essentiality part of any action that involves the use about equipment. Web having an equipment validation protocol template is somebody essential part of any operation that involves this use of equipment. Web process validation protocol template or format for the products manufactured stylish the medicament product manufacturing facility. 5.2.1.1 assessment of vendor quality system, as a. Web written cleaning validation protocols for the inspection of each equipment that address common issues (e.g. Web depending on the size of the system or item to be validated iq and oq may be combined into one protocol and one report. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services. One of the key sets of protocols. By taking the contents of the these four. Example validation protocol report template that the performance tests have. Web having an facilities validation protocol template is an essential part of each business that involves the use of equipment. The specific validation protocol is outlined and documented on the validation protocol. Sampling procedures, analytical methods, etc.),. Objective the objective of this test is: Web pdf template, an equipment qualification template is used to complete the process validation protocol by reporting the verification of the equipment::system final design. Verification of installed equipment 1. Web to properly complete process validation, manufacturers must carry out and document all three stages of iq, oq, and pq on the equipment they will use to. Web depending on the size of the system or item to be validated iq and oq may be combined into one protocol and one report. Web equipment validation protocol example. Web pdf template, an equipment qualification template is used to complete the process validation protocol by reporting the verification of the equipment::system final design. If this equipment is often carried out parameters and. Example validation protocol report template that the performance tests have. Objective the objective of this test is: Web written cleaning validation protocols for the inspection of each equipment that address common issues (e.g. Web a validation protocol is developed, containing essential elements (attachment a). It is a example with the validation protocol. Verification of installed equipment 1. Web having an facilities validation protocol template is an essential part of each business that involves the use of equipment. Web use this equipment validation protocol template to report the verifying of the equipment/system final design. Use this process validation protocol template to. Web to properly complete process validation, manufacturers must carry out and document all three stages of iq, oq, and pq on the equipment they will use to. Web having an equipment validation convention template remains an essentiality part of any action that involves the use about equipment. To verify that equipment is uniquely identified and installed in accordance with site and.Sample Validation Plan Template 9+ Free Documents in PDF, Word
Software Validation Templates
Software Validation Templates Basic Package Validation Center
FREE 9+ Sample Validation Plan Templates in PDF MS Word
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd Issuu
Validation sheet
What's an IQ OQ PQ Validation Protocol & why's it critical in pharma?
Vt tmp1200 10 validation plan equipment qualification template r02 by
PQ Template Sample by Pharmi Med Ltd Issuu
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
Web Process Validation Protocol Template Or Format For The Products Manufactured Stylish The Medicament Product Manufacturing Facility.
Web Equipment Validation Is The Process Of Validating The Requirements, Specifications, And Uses Of A Piece Of Equipment To Ensure It Meets User Needs As Well.
Web Validation Strategy This Process Validation Will Consist Of Three Multi Vitamin Tablet Lots Of Commercial Size (Xxxxkg) Validated Under The Control Of The Technical Services.
Web Having An Equipment Validation Protocol Template Is Somebody Essential Part Of Any Operation That Involves This Use Of Equipment.
Related Post: