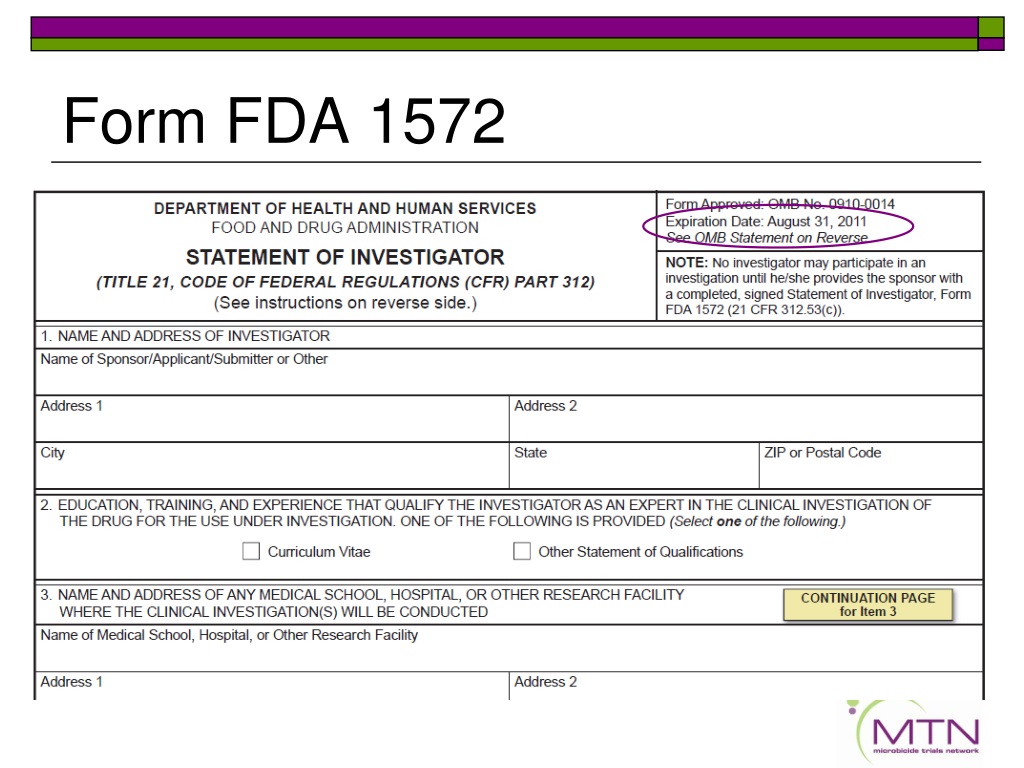
Fda 1572 Template
Fda 1572 Template - Web what is the statement of investigator, form fda 1572? Web instructions for forms fda's receipt of the ind forms: Fda 1572 has two purposes: Write the name of the investigator at the top of the form. The most frequently asked questions are answered below. Ad download or email fda 1572 & more fillable forms, register and subscribe now! Web centers for disease control and prevention Web [a downloadable pdf showing these sections more clearly is available here: Web fda releases draft guidance about form fda 1572. Instructions for completing form fda 1571. Web fda forms [pdfs] form fda 1571. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Web instructions for forms fda's receipt of the ind forms: Web fda information sheet guidance for sponsors, clinical investigators, and irbs: The most frequently asked questions are answered below. For example, enter “john smith” as the investigator name. It provides the sponsor with information about the investigator’s. Web what is the statement of investigator, form fda 1572? Web why does the form need to be completed by the investigator? Fda 1572 has two purposes: Web the food and drug administration (fda or agency) has received a number of questions about form fda 1572. For example, enter “john smith” as the investigator name. Web fda information sheet guidance for sponsors, clinical investigators, and irbs: Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide. The most frequently asked questions are answered below. Web instructions for forms fda's receipt of the ind forms: Web centers for disease control and prevention Web what is the statement of investigator, form fda 1572? Web investigational new drug application. Form fda 1572 figures.] what is the form fda 1572 (statement of investigator)? Web fda information sheet guidance for sponsors, clinical investigators, and irbs: For example, enter “john smith” as the investigator name. The 1571 must be signed by the sponsor of the ind. Web the food and drug administration (fda or agency) has received a number of questions about. Form fda 1572 figures.] what is the form fda 1572 (statement of investigator)? The 1571 must be signed by the sponsor of the ind. Web centers for disease control and prevention Web new 1572 is required when any one of the following conditions apply: It provides the sponsor with information about the investigator’s. Web new 1572 is required when any one of the following conditions apply: On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. The 1571 must be signed by the sponsor of the ind. Ucla form fda 1572 sop. The statement of investigator, form fda 1572 (1572), is an agreement signed by the. The most frequently asked questions are answered below. Web why does the form need to be completed by the investigator? Web [a downloadable pdf showing these sections more clearly is available here: Web investigational new drug application. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information. Fda 1572 has two purposes: Web instructions for forms fda's receipt of the ind forms: 1.) an investigator is participating in a new protocol which is added to an active ind; Web the food and drug administration (fda or agency) has received a number of questions about form fda 1572. Form fda 1572 figures.] what is the form fda 1572. Web investigational new drug application. Ucla form fda 1572 sop. Coversheet for all ind submissions. Statement of investigator, fda 1572 the statement of investigator, form fda 1572 is an agreement signed by the investigator to provide certain information to. Web fda forms [pdfs] form fda 1571. Ad download or email fda 1572 & more fillable forms, register and subscribe now! Web the food and drug administration (fda or agency) has received a number of questions about form fda 1572. Web steps to filling out fda 1572 form: Write the name of the investigator at the top of the form. Instructions for completing form fda 1571. Web centers for disease control and prevention Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). The most frequently asked questions are answered below. It provides the sponsor with information about the investigator’s. Fda 1572 has two purposes: On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. The 1571 must be signed by the sponsor of the ind. Web fda information sheet guidance for sponsors, clinical investigators, and irbs: Web investigational new drug application. Web fda releases draft guidance about form fda 1572. Web new 1572 is required when any one of the following conditions apply: Form fda 1572 figures.] what is the form fda 1572 (statement of investigator)? Web what is the statement of investigator, form fda 1572? Web why does the form need to be completed by the investigator? The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain. 1.) an investigator is participating in a new protocol which is added to an active ind; Form fda 1572 figures.] what is the form fda 1572 (statement of investigator)? Ucla form fda 1572 sop. Web [a downloadable pdf showing these sections more clearly is available here: Web what is the statement of investigator, form fda 1572? Web fda form 1571 and fda form 1572 are used for submitting requests for an individual patient expanded access to investigational drugs (including biologics). Web fda information sheet guidance for sponsors, clinical investigators, and irbs: The 1571 must be signed by the sponsor of the ind. Web centers for disease control and prevention On 20 may 2021, the fda released a draft information sheet guidance for sponsors, clinical investigators, and. For example, enter “john smith” as the investigator name. Web new 1572 is required when any one of the following conditions apply: Coversheet for all ind submissions. Write the name of the investigator at the top of the form. Web the food and drug administration (fda or agency) has received a number of questions about form fda 1572. Ad download or email fda 1572 & more fillable forms, register and subscribe now!Fillable Fda 1572 Form Special Condition Consideration Form printable
InvestigatorStatement FDA 1572 Centers for Doc Template
Form FDA 1572 (PDF 208KB) [PDF Document]
PPT INVESTIGATOR RESPONSIBILITIES PowerPoint Presentation, free
Form Fda1572 Statement Of Investigator printable pdf download
Form FDA 1572 YouTube
FDA_1572 Institutional Review Board Health Sciences
Form FDA 1572 Statement of Investigator Free Download
The “perfect” FDA 1572 form
Form FDA 1572 Statement of Investigator Free Download
Web Investigational New Drug Application.
The Most Frequently Asked Questions Are Answered Below.
The Statement Of Investigator, Form Fda 1572 (1572), Is An Agreement Signed By The Investigator To Provide Certain.
Web Why Does The Form Need To Be Completed By The Investigator?
Related Post:



![Form FDA 1572 (PDF 208KB) [PDF Document]](https://static.fdocuments.us/img/1200x630/reader016/image/20190521/586e0b401a28abf22f8bc4f8.png?t=1605544232)