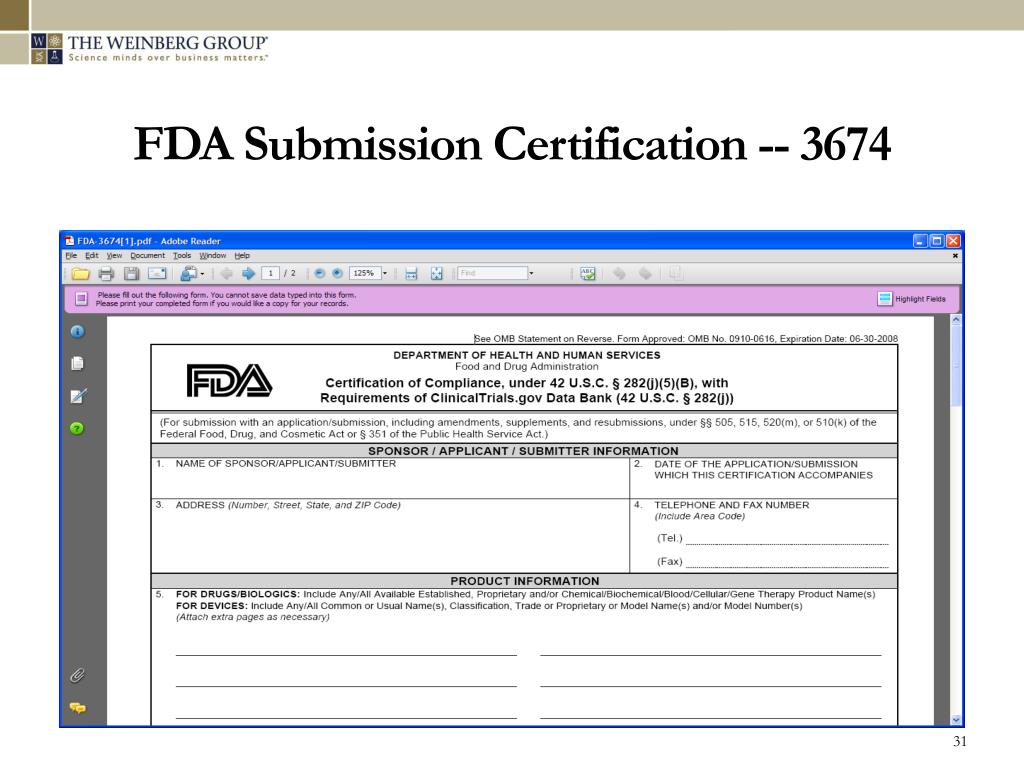
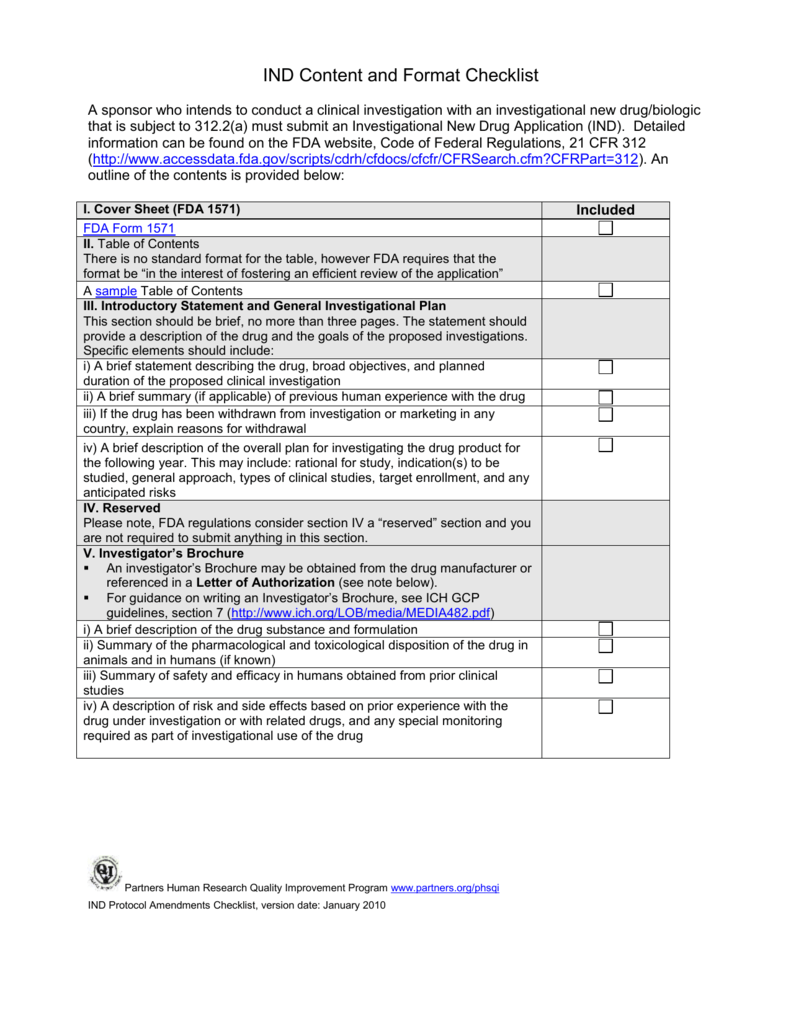
Fda Pre Submission Template
Fda Pre Submission Template - Web how to use the electronic submission template and resource (estar) pdf template. Luckily, we have organized a simple,. Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. Send and track medical device premarket submissions online: Draft guidance for industry and. Web for medical device submissions: Web ind checklist for ind submission. It includes the information that fda recommends be included in such submissions, as. However fda will not analyse any data or give a pass/fail to a result. Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references. Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Send and track medical device premarket submissions online: Web without further ado, let’s jump into the first. Web ind checklist for ind submission. Web food and drug administration [docket no. However fda will not analyse any data or give a pass/fail to a result. Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and. Additional regulatory tools and educational resources for. Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web for medical device. Web ind checklist for ind submission. Additional regulatory tools and educational resources for. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web how to use the electronic submission template and resource (estar) pdf template. Luckily, we have organized a simple,. You may not even know where to begin! Send and track medical device premarket submissions online: Luckily, we have organized a simple,. Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references. Web the presub is typically used to gain feedback on testing or protocols. Web clearly indicate what type of future submission (i.e. Web ind checklist for ind submission. It includes the information that fda recommends be included in such submissions, as. Send and track medical device premarket submissions online: Additional regulatory tools and educational resources for. Luckily, we have organized a simple,. Web how to use the electronic submission template and resource (estar) pdf template. Web for medical device submissions: Send and track medical device premarket submissions online: Web ind checklist for ind submission. Web for medical device submissions: However fda will not analyse any data or give a pass/fail to a result. Web without further ado, let’s jump into the first group. You may not even know where to begin! Send and track medical device premarket submissions online: Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. You may not even know where to begin! Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. It includes the information that fda recommends be included in such. Web food and drug administration [docket no. Luckily, we have organized a simple,. Web how to use the electronic submission template and resource (estar) pdf template. Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Web asking the right questions is crucial for medical device manufacturers seeking. However fda will not analyse any data or give a pass/fail to a result. Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s. Web how to use the electronic submission template and resource (estar) pdf template. Luckily, we have organized a simple,. Additional regulatory tools and educational resources for. Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Web for medical device submissions: Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references. Send and track medical device premarket submissions online: Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. You may not even know where to begin! Web without further ado, let’s jump into the first group. Web food and drug administration [docket no. Web ind checklist for ind submission. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Web clearly indicate what type of future submission (i.e. Draft guidance for industry and. Web the presub is typically used to gain feedback on testing or protocols. Web these template documents are meant to serve as a guide for preparation of regulatory submissions to the fda. It includes the information that fda recommends be included in such submissions, as. Web clearly indicate what type of future submission (i.e. Web the presub is typically used to gain feedback on testing or protocols. Web ind checklist for ind submission. Web this guidance is intended to represent one of several steps in meeting fda’s commitment to the development of electronic submission templates to serve as guided submission. Web without further ado, let’s jump into the first group. Web how to use the electronic submission template and resource (estar) pdf template. However fda will not analyse any data or give a pass/fail to a result. You may not even know where to begin! Web the electronic submission template, estar, is the only currently available electronic submission template to facilitate the preparation of 510 (k) electronic. Additional regulatory tools and educational resources for. Web fda forms and electronic submissions forms official fda applications and submissions forms electronic regulatory submission and review information. Luckily, we have organized a simple,. Web for medical device submissions: Web premarket submissions coversheet cover letters suggested format and address summary of safety and effectiveness data (§814.44) pma review checklist references. It includes the information that fda recommends be included in such submissions, as. Web asking the right questions is crucial for medical device manufacturers seeking feedback from the u.s.PPT Understanding the PreIDE Program FDA Perspective PowerPoint
FDA 3500A 2009 Fill and Sign Printable Template Online US Legal Forms
PPT Michael A. Swit, Esq. Vice President PowerPoint Presentation
Ind Annual Report Template
510k Cover Letter Template • Invitation Template Ideas
No.132320 FDA Presubmis… 7779 CYBERDYNE(株) 2017/01/15〜2017/01/24
A Quick & Easy Guide to FDA PreSubmissions
510(k) Submission Checklist Free Download
510k Cover Letter Template
Why the FDA PreSubmission is an Underutilized Tool
Send And Track Medical Device Premarket Submissions Online:
Draft Guidance For Industry And.
Web Food And Drug Administration [Docket No.
Web These Template Documents Are Meant To Serve As A Guide For Preparation Of Regulatory Submissions To The Fda.
Related Post: