Fda Protocol Template
Fda Protocol Template - Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a. Web to set this template's initial visibility, the |state= parameter may be used: Web 138 rows clinical trials guidance documents. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug products (hereafter referred to as products ). Web this protocol template aims to facilitate the development of two types of clinical trials involving human participants. 1) subchronic toxicity study in rodents,. The first type of trials are phase 2 and 3. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. The national institutes of health (nih) and food and drug administration (fda) developed a clinical trial protocol template with instructional and. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web clinical. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web 138 rows clinical trials guidance documents. Web this clinical trial. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web 138 rows clinical trials guidance documents. Web the food and drug administration (fda) is making available nine templates related to the submission of toxicology data. Web to set this template's. Web to set this template's initial visibility, the |state= parameter may be used: 1) subchronic toxicity study in rodents,. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Web 138 rows clinical trials guidance documents. The national institutes of health (nih) and food and drug administration (fda) developed a clinical. Web the food and drug administration (fda) is making available nine templates related to the submission of toxicology data. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. (thursday, january 19, 2023) the fda recently released an. Protocol concurrence will be issued solely based upon the information you provide in the qbr template. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web clinical trial protocols should include a clear description of trial design and patient. { {food and drug administration|state=collapsed}} to show the template collapsed, i.e.,. Web click the thumbnail to access a free template. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a. Web this template provides the food and drug administration’s. (thursday, january 19, 2023) the fda recently released an updated clinical protocol template designed for. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard format with instructional and sample text that. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good. Web 138 rows clinical. Web click the thumbnail to access a free template. Web to set this template's initial visibility, the |state= parameter may be used: Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web this clinical trial protocol template. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web the food and drug administration (fda) is making available nine templates related to the submission of toxicology data. Web 20 drug administration (fda) and sponsors or applicants. The first type of trials are phase 2 and 3. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web to set this template's initial visibility, the |state= parameter may be used: { {food and drug administration|state=collapsed}} to show the template collapsed, i.e.,. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; 1) subchronic toxicity study in rodents,. Web this protocol template aims to facilitate the development of two types of clinical trials involving human participants. Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Web fda updates the clinical protocol template. Web 138 rows clinical trials guidance documents. Web click the thumbnail to access a free template. Format and content of a rems document: Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of clinical procedures, laboratory tests, and all measures to. Clinical electronic structured harmonised protocol (cesharp) this draft guidance, when finalized, will represent the current. Protocol concurrence will be issued solely based upon the information you provide in the qbr template. Web the food and drug administration (fda) is making available nine templates related to the submission of toxicology data. Web fda updates the clinical protocol template. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a. Clinical electronic structured harmonised protocol (cesharp) this draft guidance, when finalized, will represent the current. Web the food and drug administration (fda) is making available nine templates related to the submission of toxicology data. Center for drug evaluation and research, office of regulatory policy this template is intended for interventional clinical trials of. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard format with instructional and sample text that. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in. 1) subchronic toxicity study in rodents,. Protocol concurrence will be issued solely based upon the information you provide in the qbr template. Web to set this template's initial visibility, the |state= parameter may be used: The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug products (hereafter referred to as products ). Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; The national institutes of health (nih) and food and drug administration (fda) developed a clinical trial protocol template with instructional and. { {food and drug administration|state=collapsed}} to show the template collapsed, i.e.,.Fda Recall Plan Template Fresh Fda Responds to Failures In Recall
Clinical Trial Protocol
Protocol Template 05Feb2016 508 Clinical Trial Food And Drug
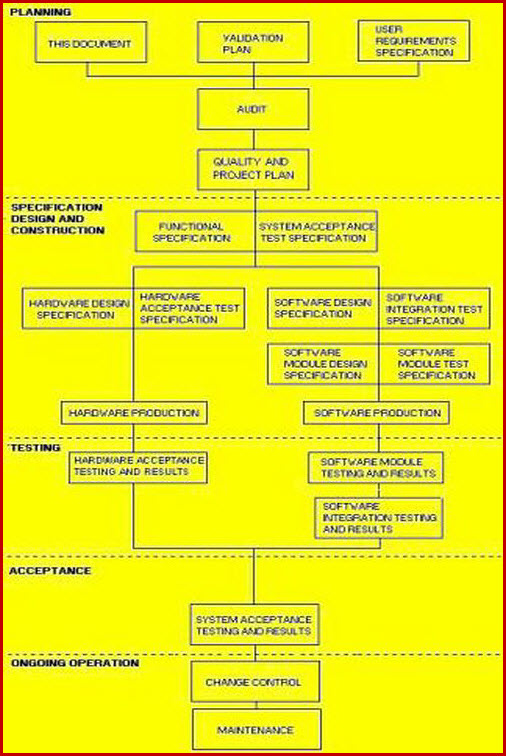
Validation Master Plan FDA EU WHO Pharma Meddevice Bio
Medical Device Process Validation Procedure ISO 13485 and FDA QSR
Medical Device Clinical Trial Protocol Template Templates MTE2MjYz
Form FDA 3486 Biological Product Deviation Report Free Download
Form FDA 0356h Application to Market a New or Abbreviated New Drug or
Nih Fda Clinical Trial Protocol Template
FDA 3500A 2009 Fill and Sign Printable Template Online US Legal Forms
Web 138 Rows Clinical Trials Guidance Documents.
Web This Clinical Trial Protocol Template Is A Suggested Format For Phase 2 And 3 Clinical Trials Funded By The National Institutes Of Health (Nih) That Are Being Conducted.
The First Type Of Trials Are Phase 2 And 3.
Guidance Documents Listed Below Represent The Agency's Current Thinking On The Conduct Of Clinical Trials, Good.
Related Post: