Fda Sop Template
Fda Sop Template - Web complete fda sop template online with us legal forms. The sops should describe a process for identifying suspect product. Drug listing regulations in 1972,. Food and drug administration's (fda) food safety plan builder (fspb) is a tool designed to assist owners/operators of food facilities with the development of food safety. Outlines the scope of the validation project and the strategy for validating the software’s. 12/11/2019 ora establishment registration and control procedure (formerly fmd# 92) page 1 of 29. Web latex template for fda sop (standard operating procedure) is there a template that could help me formatting look like an official fda sop? Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of the first items a consultant or fda auditor will request is. We often hear the acronym sop and usually associate it with. Web 30 free sop templates [word] (standard operating procedure) march 28, 2021 9 mins read. Web 30 free sop templates [word] (standard operating procedure) march 28, 2021 9 mins read. Outlines the scope of the validation project and the strategy for validating the software’s. The sops should describe a process for identifying suspect product. We often hear the acronym sop and usually associate it with. Web it is important to note the fda has no. Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to develop a mapp or a standard operating. Web food safety plan & haccp templates. Save or instantly send your ready documents. Web inspection preparedness standard operating procedure template sop number: Procedures describe who, what, when, and how. Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of the first items a consultant or fda auditor will request is. Web food safety plan & haccp templates. Web here is a sample fda software validation template: 12/11/2019 ora establishment registration and control procedure (formerly. Approved by date [signature] , principal investigator [signature]. Web inspection preparedness standard operating procedure template sop number: Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,. The sops should describe a process for identifying suspect product. Our professionally designed templates are guaranteed to make you draft an. The compliance monitoring team has created standard operating procedure templates (sops) in response to. Web complete fda sop template online with us legal forms. Save or instantly send your ready documents. Approved by date [signature] , principal investigator [signature]. Web it is important to note the fda has no requirement for what constitutes an sop or how it should be. Equipment identification and records a. Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,. Web here is a sample fda software validation template: Web inspection preparedness standard operating procedure template sop number: Web 30 free sop templates [word] (standard operating procedure) march 28, 2021 9 mins read. Food and drug administration's (fda) food safety plan builder (fspb) is a tool designed to assist owners/operators of food facilities with the development of food safety. Any optional section that does not have content will be annotated with “not applicable” or “none”. The compliance monitoring team has created standard operating procedure templates (sops) in response to. Web inspection preparedness standard. Use sops to show compliance with some of the following standards and. Drug listing regulations in 1972,. All equipment in the fda equipment. Web here is a sample fda software validation template: Fda staff manual guide (smg) 2620.2, procedure for surplus equipment 6. Creating and maintaining sops does not need to be a. Web download our free and premium templates for useful guidance and to make work much easier. Our professionally designed templates are guaranteed to make you draft an. Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to develop a mapp or a standard. Food and drug administration's (fda) food safety plan builder (fspb) is a tool designed to assist owners/operators of food facilities with the development of food safety. Web here is a sample fda software validation template: Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of. Purpose/policy this document describes the medical device single audit program (mdsap) procedures to develop, review, approve,. Web sops and sop templates are also common in government and military organizations. Any optional section that does not have content will be annotated with “not applicable” or “none”. Web latex template for fda sop (standard operating procedure) is there a template that could help me formatting look like an official fda sop? Web this manual of policies and procedures (mapp) specifies the factors to consider when determining whether to develop a mapp or a standard operating. Approved by date [signature] , principal investigator [signature]. Use sops to show compliance with some of the following standards and. Easily fill out pdf blank, edit, and sign them. All equipment in the fda equipment. Our professionally designed templates are guaranteed to make you draft an. The sops should describe a process for investigating suspect product that has been detected/identified,. Web fda sends warning letters to companies for not having adequate sops, as well as not following them. Web food safety plan & haccp templates. Equipment identification and records a. Drug listing regulations in 1972,. Web download our free and premium templates for useful guidance and to make work much easier. 12/11/2019 ora establishment registration and control procedure (formerly fmd# 92) page 1 of 29. Procedures describe who, what, when, and how. Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of the first items a consultant or fda auditor will request is. Web here is a sample fda software validation template: The sops should describe a process for identifying suspect product. Save or instantly send your ready documents. Web 30 free sop templates [word] (standard operating procedure) march 28, 2021 9 mins read. 12/11/2019 ora establishment registration and control procedure (formerly fmd# 92) page 1 of 29. Outlines the scope of the validation project and the strategy for validating the software’s. Our professionally designed templates are guaranteed to make you draft an. Use sops to show compliance with some of the following standards and. Easily fill out pdf blank, edit, and sign them. Web it is important to note the fda has no requirement for what constitutes an sop or how it should be formatted, yet one of the first items a consultant or fda auditor will request is. The sops should describe a process for investigating suspect product that has been detected/identified,. Creating and maintaining sops does not need to be a. Web download our free and premium templates for useful guidance and to make work much easier. Procedures describe who, what, when, and how. The compliance monitoring team has created standard operating procedure templates (sops) in response to. Approved by date [signature] , principal investigator [signature]. All equipment in the fda equipment.Operating Procedure Example Sample Templates Standard FDA Sop inside
Army Sop Template
Fda Recall Plan Template Fresh Fda Responds to Failures In Recall
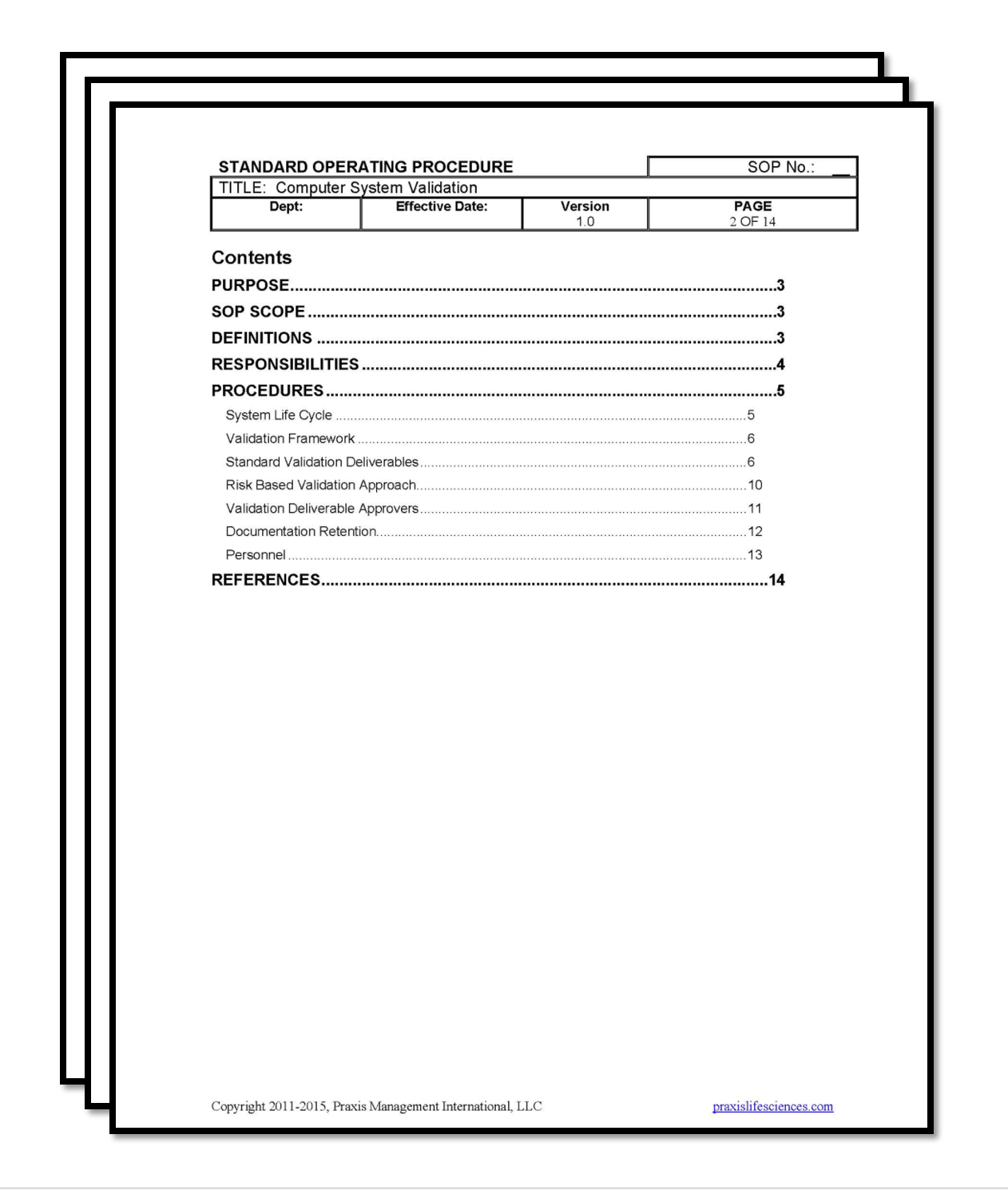
Computer System Validation SOP Validation Center
SOP For Corrective Action and Preventive Actions Pharmaceutical
Complaint Handling Procedure
PRODUCT RECALLS SOP Template PH32 GMP, QSR & ISO Compliance
37 Best Standard Operating Procedure (SOP) Templates
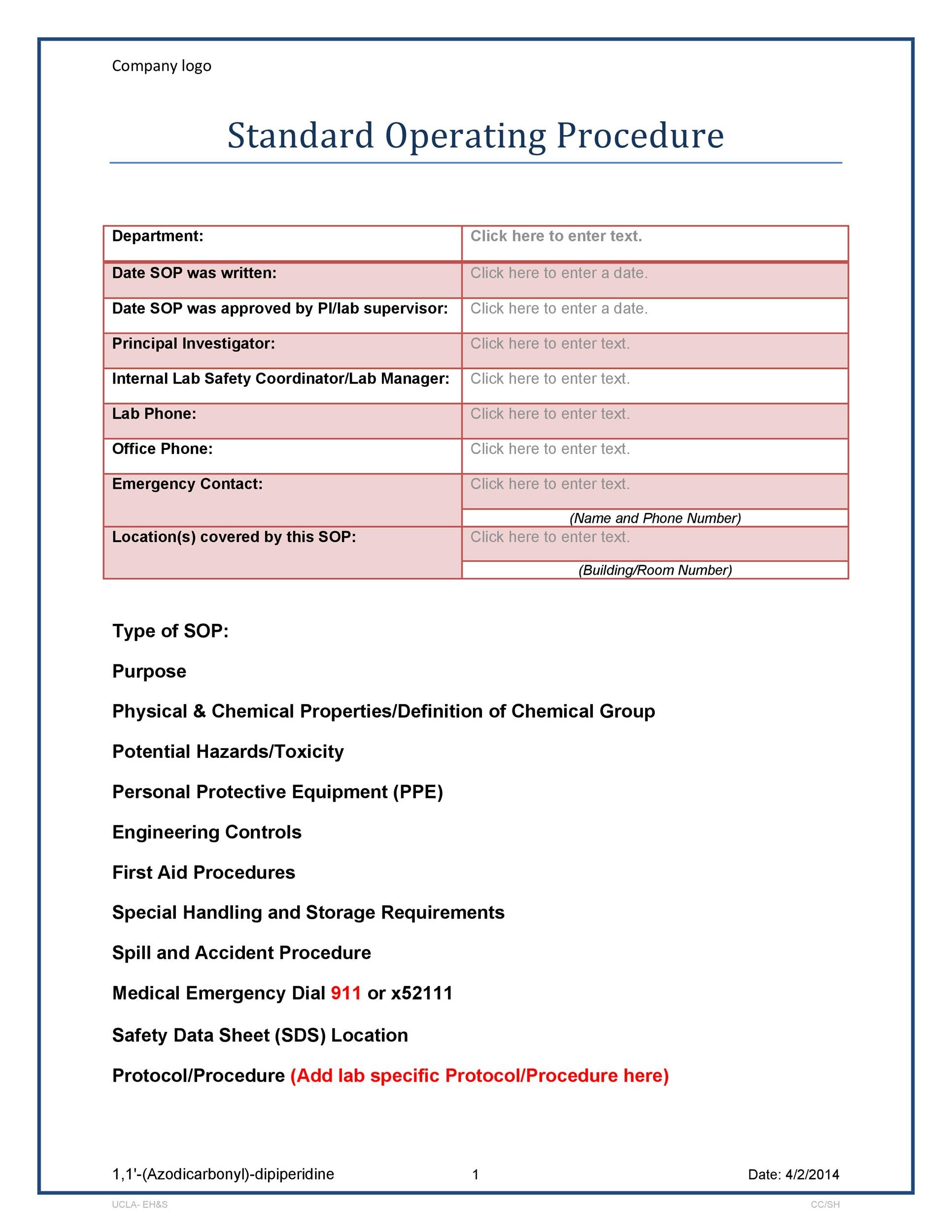
How to write a standard operating procedure sop
21 CFR Part 11 Assessment Template
Fda Staff Manual Guide (Smg) 2620.2, Procedure For Surplus Equipment 6.
Web Inspection Preparedness Standard Operating Procedure Template Sop Number:
Web Here Is A Sample Fda Software Validation Template:
Web Food And Drug Administration Office Of Regulatory Affairs Ora Laboratory Manual Volume Ii Document Number:
Related Post: