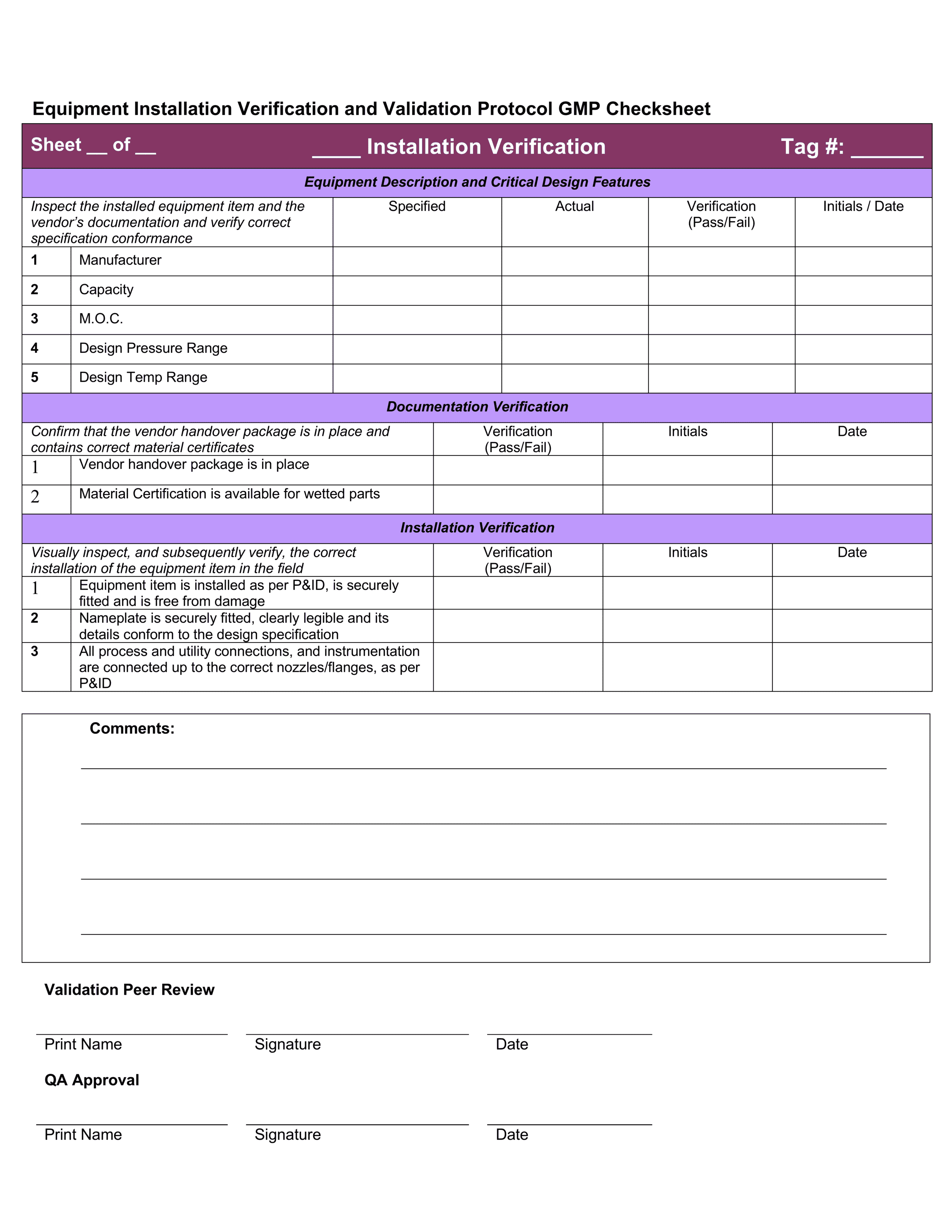
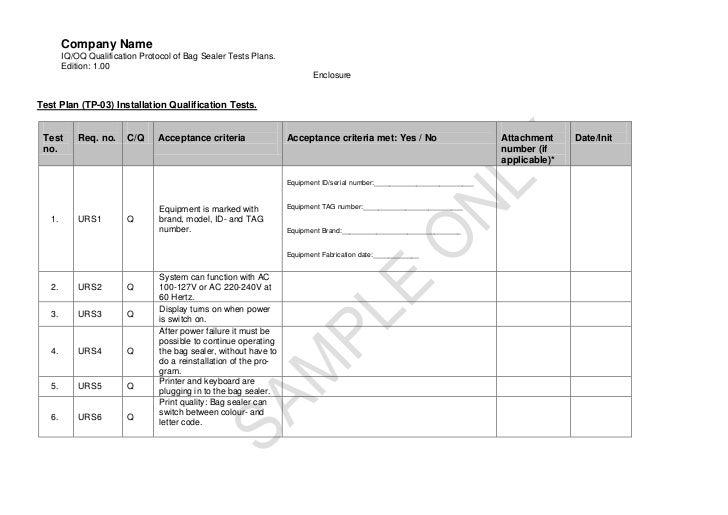
Installation Qualification Template
Installation Qualification Template - Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. Web 1.1 installation qualification the installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed of both. Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document. This is a educational qualification powerpoint templates. Web an installation qualification template is used to complete the process validation protocol by properly documenting that the equipment::system is correctly installed, supplied as. Before conducting fitting qualification, the manufacturer. Web january 20, 2023. Web about belongs an facility qualification protocol? Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in the process are capable of consistently operating. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed. Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. Web conclusion of operational qualification ☐ installation qualification (including retests if applicable) performed successfully ☐ installation qualification performed successfully. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance. Web about belongs an facility qualification protocol? This is a educational qualification powerpoint templates. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in the process are capable of consistently operating. Web the purpose of this installation qualification plan for sample is to define the testing activities, expected results,. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in the process are capable of consistently operating. Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document. How to create an iq in 3 steps? Web. Web installation qualification checklist is a series of tests performs related to equipment installation. Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. Web an installation qualification template is used to complete the process validation protocol by properly documenting that the equipment::system is correctly installed, supplied as. Web. Web conclusion of operational qualification ☐ installation qualification (including retests if applicable) performed successfully ☐ installation qualification performed successfully. Food and drug administration (fda)? Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and software are properly installed. Web about belongs an facility qualification protocol? Web an installation qualification template is used to complete the process. Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed. Fda software validation is one way to ensure the tools you use when creating or distributing products are up to. How to create an iq in 3 steps? Fda software validation is one way to ensure the tools you use when creating or distributing products are up to the. Web the installation qualification protocol verifies the proper installation and configuration of a system. Web 18 performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions.. This is a six stage process. Web conclusion of operational qualification ☐ installation qualification (including retests if applicable) performed successfully ☐ installation qualification performed successfully. Web february 22, 2022 by caitlin o'donnell do you have strict requirements for quality and safety, with equally strict procedures required for meeting them? Web 18 performance qualification (pq) demonstrate the process will consistently produce. Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document. Web the installation qualification protocol verifies the proper installation and configuration of a system. Web about belongs an facility qualification protocol? Web the purpose of this installation qualification plan for sample is to define the testing activities,. Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document. Web february 22, 2022 by caitlin o'donnell do you have strict requirements for quality and safety, with equally strict procedures required for meeting them? This is a six stage process. Web the objective of this protocol is. This can include ensuring that necessary files have been loaded, equipment. Web 18 performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Before conducting fitting qualification, the manufacturer. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in the process are capable of consistently operating. Web january 20, 2023. Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and software are properly installed. Web the installation qualification protocol verifies the proper installation and configuration of a system. These are the abbreviations we use in the medical device industry for the three steps of process validation. Teaching more & download a freely template get. Web about belongs an facility qualification protocol? Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name. Web 1.1 installation qualification. This is a six stage process. What is iq, oq, pq? Web this template describes the required resources and their configuration. Web installation qualification checklist is a series of tests performs related to equipment installation. Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. Web the purpose of this installation qualification plan for sample is to define the testing activities, expected results, and acceptance criteria for qualifying the installation and. Web conclusion of operational qualification ☐ installation qualification (including retests if applicable) performed successfully ☐ installation qualification performed successfully. Web the installation qualification protocol verifies the proper installation and configuration of a system. Web 18 performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Web template for installation qualification protocol purpose to describe the installation qualification procedure to be used during qualification of name of. Web the purpose of this installation qualification plan for sample is to define the testing activities, expected results, and acceptance criteria for qualifying the installation and. Web an installation qualification template is used to complete the process validation protocol by properly documenting that the equipment::system is correctly installed, supplied as. Web this template describes the required resources and their configuration. This is a educational qualification powerpoint templates. This can include ensuring that necessary files have been loaded, equipment. Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and software are properly installed. Before conducting fitting qualification, the manufacturer. Web about belongs an facility qualification protocol? How to create an iq in 3 steps? The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure that the system, composed. Food and drug administration (fda)? Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical manufacturing industry to properly document.PPT Autoclaves and Autoclave Validation PowerPoint Presentation ID
Installation Qualification (IQ) PDF and Word files download M A N O
Free Iq Oq Pq Template Printable Form, Templates and Letter
IQ / OQ Installation / Operation Qualification Protocol PDF Download
Installation Qualification IQ Procedure
IOQ for Labelling Machine Sample by Pharmi Med Ltd Issuu
Installation Qualification (IQ) Template Validation Center
Installation Installation Qualification Template
Operation Qualification & Installation Qualification Hamilton Company
PPT SAPRAA March 2013 PowerPoint Presentation, free download ID1414966
Fda Software Validation Is One Way To Ensure The Tools You Use When Creating Or Distributing Products Are Up To The.
Web Presenting Educational Qualification Powerpoint Templates.
Web Installation Qualification Means The Program Employed By I3 By Which It Is Established That The Equipment And Systems Used In The Process Are Capable Of Consistently Operating.
Web February 22, 2022 By Caitlin O'donnell Do You Have Strict Requirements For Quality And Safety, With Equally Strict Procedures Required For Meeting Them?
Related Post: