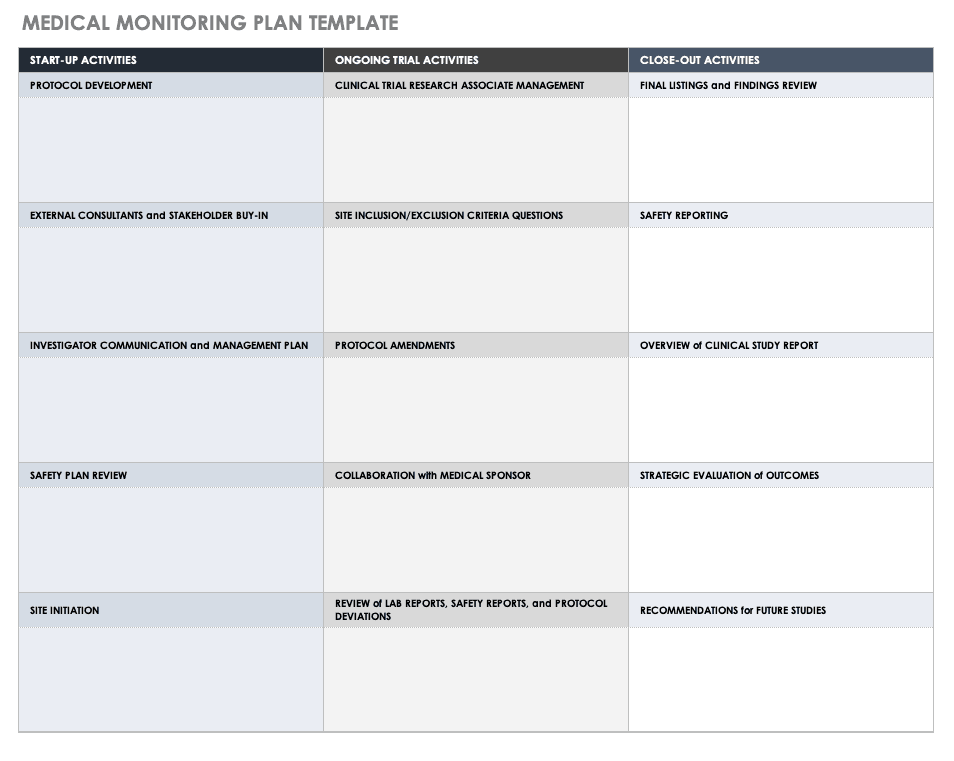
Medical Monitoring Plan Template
Medical Monitoring Plan Template - Best practice recommendations • review this draft. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web nidcr clinical monitoring guidelines. Ad learn how philips patient monitoring can help unlock capabilities across your system. Which clinical studies require a. Web example data and safety monitoring plan (dsmp) independent monitor. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Web clinical monitoring plan template. Web 1.1 purpose the california code of regulations, title 8 requires that employees of california state university, chico (the “university”) with exposure potential to certain. Web nimh clinical monitoring plan template [word] this template provides a recommended structure for a plan to conduct internal or independent review of good clinical practices. Who needs a data and safety monitoring plan (dsmp) the content of a dsmp. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. 14 march 2019page 1 of 17 n2 quality committee guidance for developing monitoring plan s introduction the. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams.. Best practice recommendations • review this draft. Web 1.1 purpose the california code of regulations, title 8 requires that employees of california state university, chico (the “university”) with exposure potential to certain. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Best practice recommendations • review this draft. Web. Web this webpage provides guidance for niddk grant applicants on. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Web clinical study report template. 14 march 2019page 1 of 17 n2 quality committee guidance for developing monitoring plan s introduction the. Web nidcr clinical monitoring. Web 1.1 purpose the california code of regulations, title 8 requires that employees of california state university, chico (the “university”) with exposure potential to certain. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Web nimh clinical monitoring plan template [word] this template provides a recommended structure for a plan to conduct. Find out how our patient monitoring products can help deliver operational efficiencies. Who needs a data and safety monitoring plan (dsmp) the content of a dsmp. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Throughout the template there are suggested. Web clinical study report template. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies. Best practice recommendations • review this draft. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web monitoring plan template page 1of 17 version date: Ad learn how philips patient monitoring can help unlock capabilities across your system. Also, we have included a proposed structure for a. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these.. Also, we have included a proposed structure for a. Web nimh clinical monitoring plan template [word] this template provides a recommended structure for a plan to conduct internal or independent review of good clinical practices. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Web. Web purpose {explain purpose of monitoring plan, for example:} the purpose of this monitoring plan is to describe the rationale and process for the collection, recording,. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Web clinical study report template. Web designated medical monitor. Web monitoring plan template page 1of 17 version. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Find out how our patient monitoring products can help deliver operational efficiencies. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. 14 march 2019page 1 of 17 n2 quality committee guidance for developing monitoring plan s introduction the. Exampledata and safety monitoring plan (dsmp)independent monitor. Web nidcr clinical monitoring guidelines. Web purpose {explain purpose of monitoring plan, for example:} the purpose of this monitoring plan is to describe the rationale and process for the collection, recording,. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Web this webpage provides guidance for niddk grant applicants on. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Web nimh clinical monitoring plan template [word] this template provides a recommended structure for a plan to conduct internal or independent review of good clinical practices. Web designated medical monitor. Web monitoring plan template page 1of 17 version date: Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Throughout the template there are suggested. Best practice recommendations • review this draft. Web example data and safety monitoring plan (dsmp) independent monitor. Also, we have included a proposed structure for a. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web purpose {explain purpose of monitoring plan, for example:} the purpose of this monitoring plan is to describe the rationale and process for the collection, recording,. Ad learn how philips patient monitoring can help unlock capabilities across your system. Which clinical studies require a. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Web 1.1 purpose the california code of regulations, title 8 requires that employees of california state university, chico (the “university”) with exposure potential to certain. Web nimh clinical monitoring plan template [word] this template provides a recommended structure for a plan to conduct internal or independent review of good clinical practices. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web clinical study report template. Web nidcr clinical monitoring guidelines. Web designated medical monitor. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Guidance for assisting grantees conducting or planning to conduct clinical trials, has developed these. Web example data and safety monitoring plan (dsmp) independent monitor. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical trials or research. Throughout the template there are suggested.Monitoring Plan Template
Care Physical health International Cat Care
Example of Monitoring Sheet PDF Teachers Curriculum
The enchanting The Basics Of Clinical Trial Centralized Monitoring For
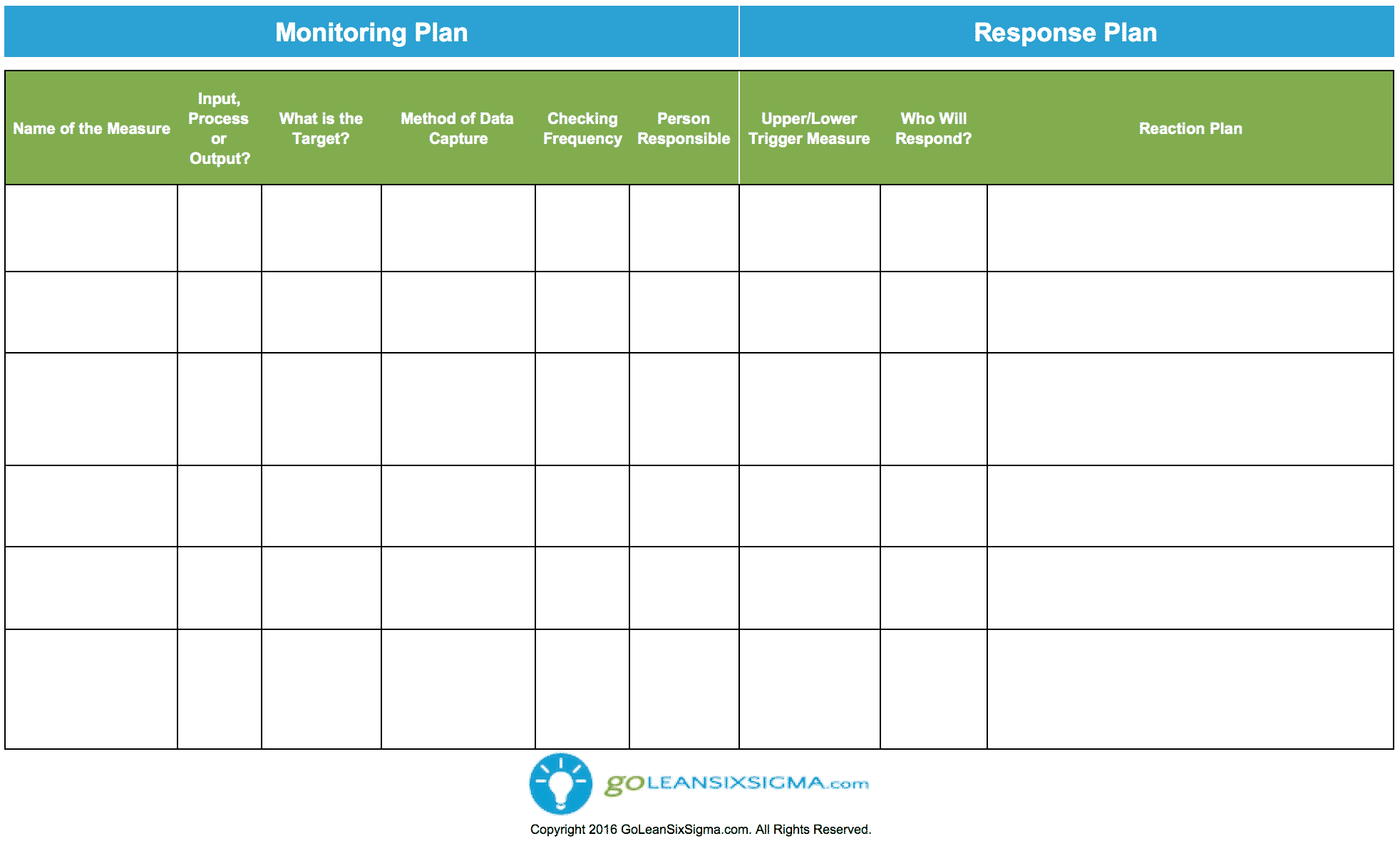
Monitoring & Response Plan Template & Example
Patient Tracking Spreadsheet —
3.8. Template for monitoring plan
hospital compliance plan Jennies Blog hospital risk management
Monitoring Report Template Clinical Trials ] Saving Lives Pertaining
Free Medical Form Templates Smartsheet
Who Needs A Data And Safety Monitoring Plan (Dsmp) The Content Of A Dsmp.
Web Instructions This Template Is A Suggested Format For A Monitoring Plan Developed By Tb Survey Teams.
Find Out How Our Patient Monitoring Products Can Help Deliver Operational Efficiencies.
14 March 2019Page 1 Of 17 N2 Quality Committee Guidance For Developing Monitoring Plan S Introduction The.
Related Post:







![Monitoring Report Template Clinical Trials ] Saving Lives Pertaining](https://pray.gelorailmu.com/wp-content/uploads/2020/01/monitoring-report-template-clinical-trials-saving-lives-pertaining-to-monitoring-report-template-clinical-trials-1603x2048.png)