Monitoring Plan Template For Clinical Trials
Monitoring Plan Template For Clinical Trials - Web such trials usually involve a large number of participants followed for longer periods of treatment exposure to monitor adverse events and to collect information that. After nine years in draft form and 127 comments received from stakeholders, the fda has finalized its guidance on informed consent, adding a. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Web pk !ýb‰ î : Also, we have included a proposed structure for a. Best practice recommendations review this draft. Guidance for clinical research associates responsible for preparing a clinical monitoring plan. Web this guidance was created to aid investigators developing a data and safety monitoring plan (dsmp) to ensure the safety of research participants and to protect the validity and. The clinical trial monitors will conduct monitoring. Best practice recommendations review this draft. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Best practice recommendations review this draft. It also outlines the responsibilities. The clinical trial monitors will conduct monitoring. Web august 21, 2023. Web applicants conducting phase 2 or 3 clinical trials that require investigational new drug applications (ind) or investigational device exemption (ide) applications can use an. Web clinical monitoring plan template. Web data and safety monitoring plan template and guidelines (delete this) preface investigators should consider using this template when developing t he data. Web nidcr clinical monitoring guidelines. Guidance for. Web monitoring is the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in accordance with the protocol, standard operating. Web clinical trial monitoring requires data collection and analysis throughout a project to ensure appropriateness of the research and project design, validity and integrity of the. Guidance document that provides. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. This week’s pipeline features trial approvals for alzheimer’s disease, systemic lupus erythematosus and. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Guidance for clinical research associates responsible for preparing a. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Web this guidance was created to aid investigators developing a data and safety monitoring plan (dsmp). Web nidcr clinical monitoring guidelines. Web drug & device pipeline news. It also outlines the responsibilities. Web monitoring is the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in accordance with the protocol, standard operating. Web the national institute of mental health (nimh) has developed the following guidance for. This week’s pipeline features trial approvals for alzheimer’s disease, systemic lupus erythematosus and. Web this guidance was created to aid investigators developing a data and safety monitoring plan (dsmp) to ensure the safety of research participants and to protect the validity and. Web clinical trial monitoring requires data collection and analysis throughout a project to ensure appropriateness of the research. Best practice recommendations review this draft. Web pk !ýb‰ î : Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. The clinical trial monitors will conduct monitoring. Web this is an ms word template to use as a starting point for preparing a medical monitoring plan for clinical. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Best practice recommendations review this draft. Web monitoring is the act of. Monitoring agreement for local independent safety monitor template. The clinical trial monitors will conduct monitoring. Web this document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. Web data and safety monitoring plan template and guidelines (delete this) preface investigators should consider using this template when developing t he data. It. Web this guidance was created to aid investigators developing a data and safety monitoring plan (dsmp) to ensure the safety of research participants and to protect the validity and. Web drug & device pipeline news. Best practice recommendations review this draft. Monitoring agreement for local independent safety monitor template. Web clinical trial monitoring requires data collection and analysis throughout a project to ensure appropriateness of the research and project design, validity and integrity of the. The clinical trial monitors will conduct monitoring. Web applicants conducting phase 2 or 3 clinical trials that require investigational new drug applications (ind) or investigational device exemption (ide) applications can use an. This week’s pipeline features trial approvals for alzheimer’s disease, systemic lupus erythematosus and. Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web nidcr clinical monitoring guidelines. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken. Best practice recommendations review this draft. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web monitoring is the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in accordance with the protocol, standard operating. Web such trials usually involve a large number of participants followed for longer periods of treatment exposure to monitor adverse events and to collect information that. Web this document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web august 21, 2023. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. It also outlines the responsibilities. Web drug & device pipeline news. Web monitoring is the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in accordance with the protocol, standard operating. Best practice recommendations review this draft. Web this document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web august 21, 2023. Also, we have included a proposed structure for a. Web such trials usually involve a large number of participants followed for longer periods of treatment exposure to monitor adverse events and to collect information that. Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Web applicants conducting phase 2 or 3 clinical trials that require investigational new drug applications (ind) or investigational device exemption (ide) applications can use an. After nine years in draft form and 127 comments received from stakeholders, the fda has finalized its guidance on informed consent, adding a. Web this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web this guidance was created to aid investigators developing a data and safety monitoring plan (dsmp) to ensure the safety of research participants and to protect the validity and. The clinical trial monitors will conduct monitoring. This week’s pipeline features trial approvals for alzheimer’s disease, systemic lupus erythematosus and. Web clinical monitoring plan template describes how you will go about monitoring the conduct of your trial and justifies the approach taken.Monitoring Report Template Clinical Trials Sample Design Templates
Site Monitoring Plan Template PDF Clinical Trial Business
Monitoring Report Template Clinical Trials (2) TEMPLATES EXAMPLE
Free Clinical Trial Templates Smartsheet
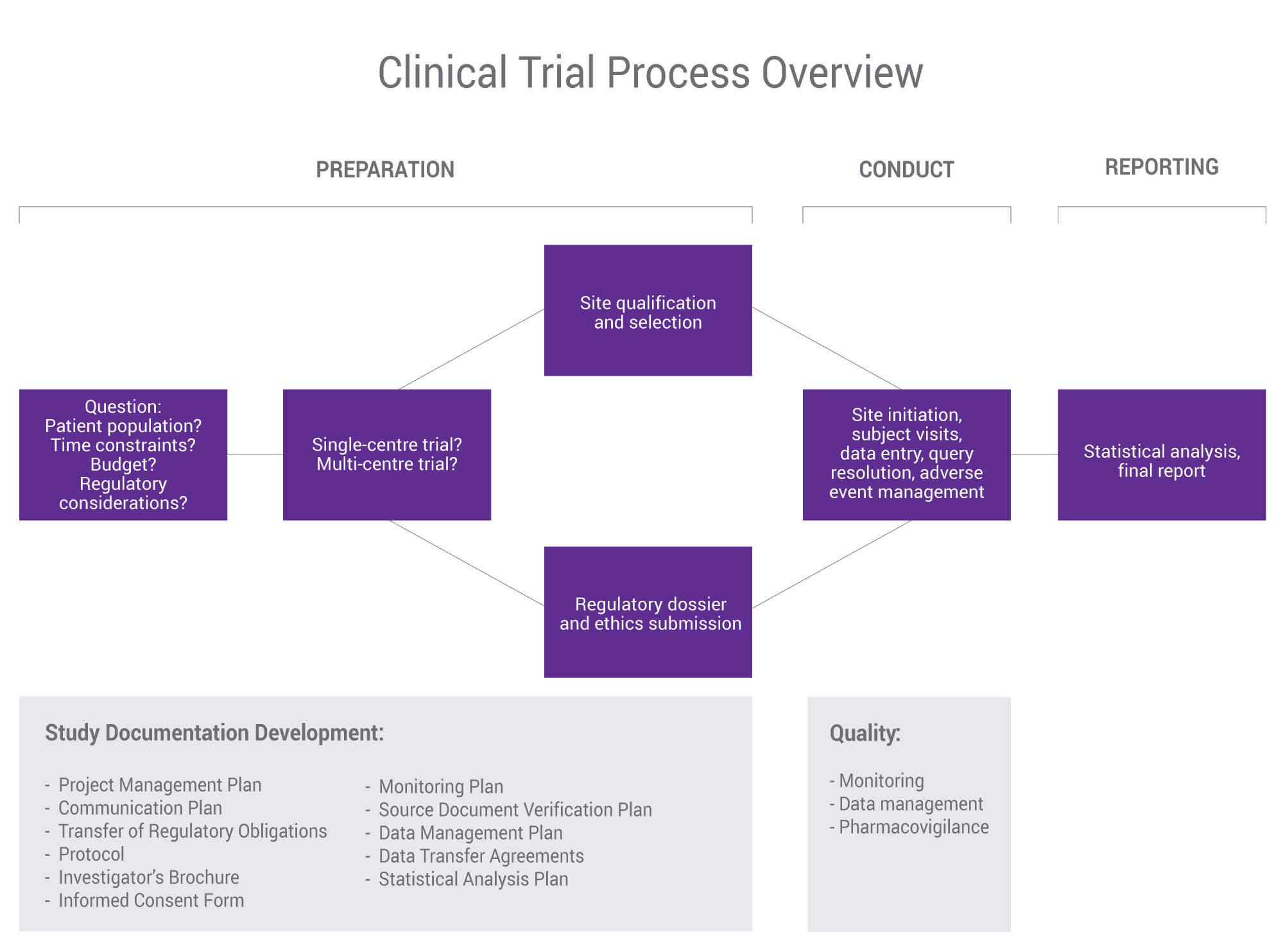
3.8. Template for monitoring plan
Monitoring Report Template Clinical Trials (5) TEMPLATES EXAMPLE
Monitoring Report Template Clinical Trials
Management Plans Al Trial Project Plan Example Template with regard to
Monitoring Report Template Clinical Trials
The Basics Of Clinical Trial Centralized Monitoring with regard to
Web Investigators Should Consider Using This Template When Developing The Data And Safety Monitoring Plan (Dsmp) For Clinical Studies Funded By The National Institute Of Arthritis.
Guidance For Clinical Research Associates Responsible For Preparing A Clinical Monitoring Plan.
Web Nidcr Clinical Monitoring Guidelines.
Guidance Document That Provides Detailed Descriptions Of The Nidcr Clinical Monitoring Processes.
Related Post: