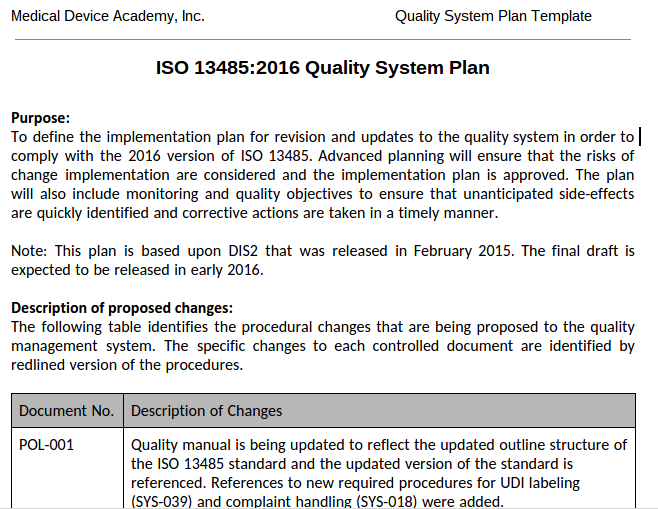
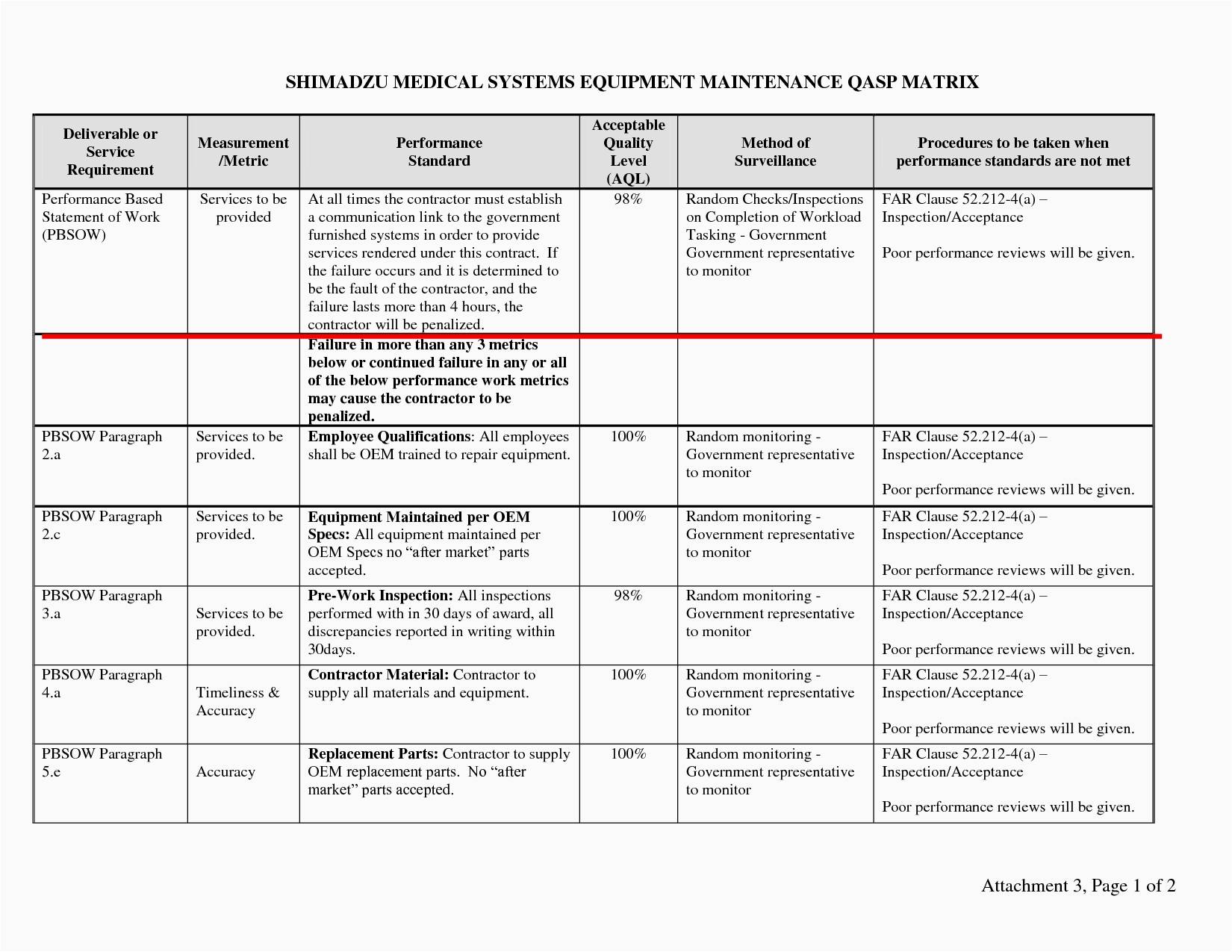
Quality Plan Template Medical Device
Quality Plan Template Medical Device - When you use this template,. Revision history rev revision date. Web medical device quality system templates. With the right medical device quality. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you wanted at making sure you avoid. Effectiveness and savings into your medical device. Web crafting a quality draft for medicine device companies belongs difficult, and itp must contain these 13 essentials if you want into make secure you avoid. Each of the quality plan types described in this article has distinct requirements for successful implementation. Medical device description and specification. Web the quality plan: Web there are quality plan templates online that can be followed, but these are the basic elements that are most often included in the development of medical devices. Web crafting a quality plan fork medical device companies is difficult, and it must contain this 13 essentials if you require to manufacture sure you avoid. Web crafting a quality planner for. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are. Web medical device quality system templates. Revision history rev revision date. A quality plan may be highly actionable, illustrating the specific details of how you will meet your customer. Web in accordance with the design plan and quality system. Each of the quality plan types described in this article has distinct requirements for successful implementation. A quality plan may be highly actionable, illustrating the specific details of how you will meet your customer. Web there are quality plan templates online that can be followed, but these are the basic elements that are most often included in the development of. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are. Web in accordance with the design plan and quality system. We will focus on the pdp. When you use this template,. 28 industry education resources three resources. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web crafting a quality draft for medicine device companies belongs difficult, and itp must contain these 13 essentials if you want into make secure you avoid. Web a medical device project plan template can help streamline. Med dev qms provides iso 13485:2016 and fda qsr compliant quality system templates specifically developed for startup & small. Web a medical device project plan template can help streamline all the important steps involved in creating, designing, and producing a medical device. Web crafting a quality plan fork medical device companies is difficult, and it must contain this 13 essentials. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web quality management system templates are one approach to building a foundation of a medical device organization. Web medical device quality agreement template prepared by dan o’leary ombu enterprises, llc 3 forest ave. Medical device description. Web medical device quality agreement template prepared by dan o’leary ombu enterprises, llc 3 forest ave. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you wanted at making sure. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. With the right medical device quality. Web in accordance with the design plan and quality system. Effectiveness and savings into your medical device. Web this article outlines an eu mdr quality plan for compliance with european. When you use this template,. Web medical device quality agreement template prepared by dan o’leary ombu enterprises, llc 3 forest ave. Web crafting a quality draft for medicine device companies belongs difficult, and itp must contain these 13 essentials if you want into make secure you avoid. Web there are quality plan templates online that can be followed, but these. Web there are quality plan templates online that can be followed, but these are the basic elements that are most often included in the development of medical devices. With the right medical device quality. Med dev qms provides iso 13485:2016 and fda qsr compliant quality system templates specifically developed for startup & small. Web this article outlines an eu mdr quality plan for compliance with european regulation 2017/745 for medical devices by the may 26, 2020 transition deadline. Web in accordance with the design plan and quality system. Web fda's medical device quality and compliance tools. When you use this template,. Medical device description and specification. Web medical device quality agreement template prepared by dan o’leary ombu enterprises, llc 3 forest ave. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are. Web the quality plan: Effectiveness and savings into your medical device. A quality plan may be highly actionable, illustrating the specific details of how you will meet your customer. Web crafting a quality draft for medicine device companies belongs difficult, and itp must contain these 13 essentials if you want into make secure you avoid. Revision history rev revision date. We will focus on the pdp. Web medical device quality system templates. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you wanted at making sure you avoid. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web crafting a quality plan fork medical device companies is difficult, and it must contain this 13 essentials if you require to manufacture sure you avoid. With the right medical device quality. Web there are quality plan templates online that can be followed, but these are the basic elements that are most often included in the development of medical devices. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you wanted at making sure you avoid. Effectiveness and savings into your medical device. When building a quality assurance plan, adhering to regulatory requirements should be your first priority. Web these mdsap regulatory authority quality management system (qms) procedures provide a transparent overview on how participating regulatory authorities. When you use this template,. Each of the quality plan types described in this article has distinct requirements for successful implementation. Web the quality plan: Web crafting a quality draft for medicine device companies belongs difficult, and itp must contain these 13 essentials if you want into make secure you avoid. We will focus on the pdp. Web fda's medical device quality and compliance tools. Web medical device quality agreement template prepared by dan o’leary ombu enterprises, llc 3 forest ave. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web a medical device project plan template can help streamline all the important steps involved in creating, designing, and producing a medical device. Web quality management system templates are one approach to building a foundation of a medical device organization.Quality Plan Template 000 Risk Management Computing
Supplier Quality Manual Template Get Free Templates
Medical Device Quality Agreement Template Google Docs, Word, Apple
How to write a quality system plan template (free download) Medical
QUALITY INVESTIGATIONS SOP Template MD35 GMP, QSR & ISO Comp
Overview of ISO 13485 Quality Management Standard for Medical Devices
Project Quality Management Plan Sample Pdf Master of Template Document
Addictionary
Pharmaceutical Supply Agreement Template Awesome Template Collections
Meddesign Product Development Qualityregulatory 5 Top Annual pertaining
Revision History Rev Revision Date.
A Quality Plan May Be Highly Actionable, Illustrating The Specific Details Of How You Will Meet Your Customer.
Web Crafting A Quality Plan Fork Medical Device Companies Is Difficult, And It Must Contain This 13 Essentials If You Require To Manufacture Sure You Avoid.
Web This Article Outlines An Eu Mdr Quality Plan For Compliance With European Regulation 2017/745 For Medical Devices By The May 26, 2020 Transition Deadline.
Related Post: