Regulatory Strategy Template For Medical Devices
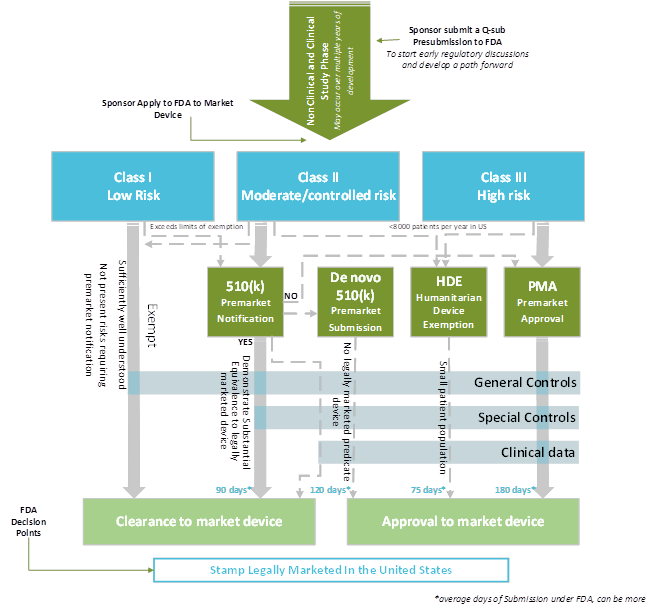
Regulatory Strategy Template For Medical Devices - This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. Class i, class ii or class iii, based on the level of control necessary to provide reasonable assurance of its safety and. Frameworks, tools & templates to improve your strategic planning capability. Explore regulatory options to streamline and modernize timely implementation of A regulatory strategy is more than just picking a. Ad management consultants offering the world's best business toolkits, frameworks & templates. The regulatory, quality, compliance and strategy experts your life science you need. Determining a cost/return on investment for. These are the most important ones so you should probably get started. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines, budgets, resources and. Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Web #1 does anyone have a regulatory plan template that they would like to share? Web each device is assigned to one of three regulatory classes: Determining a cost/return on investment for. The regulatory, quality,. Web planning your medical device global market regulatory strategy. Web each device is assigned to one of three regulatory classes: Web these include your intended use (super important) and your mdr classification document, among others. This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. Class i, class ii or. Ad management consultants offering the world's best business toolkits, frameworks & templates. This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. These are the most important ones so you should probably get started. Web a new requirement for a manufacturer of medical devices and in vitro diagnostics (ivds) is. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. Web background where does regulatory strategy fit in product development? Web when we are speaking about. Web an effective regulatory compliance strategy for medical devices must contain a number of elements, including: Contains nonbinding recommendations (version october 6, 2021) 2 Frameworks, tools & templates to improve your strategic planning capability. The regulatory, quality, compliance and strategy experts your life science you need. Ad management consultants offering the world's best business toolkits, frameworks & templates. Web planning your medical device global market regulatory strategy. Web each device is assigned to one of three regulatory classes: Explore regulatory options to streamline and modernize timely implementation of Web an effective regulatory compliance strategy for medical devices must contain a number of elements, including: Establish a robust medical device patient safety net in the united states 2. Web background where does regulatory strategy fit in product development? A regulatory strategy is more than just picking a. Web in this article i'll address design planning, the associated regulatory requirements, and how proper planning should be carried out to control the design of. The regulatory, quality, compliance and strategy experts your life science you need. Web 1 additionally, to. A regulatory strategy is more than just picking a. I am looking for templates for us, canada and eu regulatory compliance for. Web feb 18, 2021 strategy for regulatory compliance for mdr with template as european regulatory compliance becomes more complicated many medical device. Ad management consultants offering the world's best business toolkits, frameworks & templates. Contains nonbinding recommendations (version. I am looking for templates for us, canada and eu regulatory compliance for. Explore regulatory options to streamline and modernize timely implementation of Ad click now to learn more about rca's global consulting network and service solutions. Our strategic assessments of regulatory requirements address business needs for medical device, in vitro diagnostic and combination product. Web background where does regulatory. This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. I am looking for templates for us, canada and eu regulatory compliance for. A regulatory strategy is more than just picking a. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines,. Frameworks, tools & templates to improve your strategic planning capability. Our strategic assessments of regulatory requirements address business needs for medical device, in vitro diagnostic and combination product. Ad click now to learn more about rca's global consulting network and service solutions. Ad management consultants offering the world's best business toolkits, frameworks & templates. Explore regulatory options to streamline and modernize timely implementation of Web each device is assigned to one of three regulatory classes: Web these include your intended use (super important) and your mdr classification document, among others. Web when we are speaking about regulatory strategy for medical devices, three major strategies can cause delays in obtaining approval to market in any country. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. I am looking for templates for us, canada and eu regulatory compliance for. Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Web planning your medical device global market regulatory strategy. Class i, class ii or class iii, based on the level of control necessary to provide reasonable assurance of its safety and. Web an effective regulatory compliance strategy for medical devices must contain a number of elements, including: Department of health and human services, food and drug. Web strategy and implementation plan. Web feb 18, 2021 strategy for regulatory compliance for mdr with template as european regulatory compliance becomes more complicated many medical device. These are the most important ones so you should probably get started. Web 1 additionally, to successfully navigate the complex regulatory system, recognize and respect factors such as development timelines, budgets, resources and. Determining a cost/return on investment for. I am looking for templates for us, canada and eu regulatory compliance for. A regulatory strategy is more than just picking a. This comprises regulatory provisions like registration requirements and processes, but also (not legally binding) guidelines as well as predictive approval. Web an effective regulatory compliance strategy for medical devices must contain a number of elements, including: Indications for use (ifu) your team should develop an ifu (a basic description of how the device is intended to be used), and should include:. Web strategy and implementation plan. Web in this article i'll address design planning, the associated regulatory requirements, and how proper planning should be carried out to control the design of. Web a new requirement for a manufacturer of medical devices and in vitro diagnostics (ivds) is to have a strategy for regulatory compliance. Web #1 does anyone have a regulatory plan template that they would like to share? The regulatory, quality, compliance and strategy experts your life science you need. Establish a robust medical device patient safety net in the united states 2. Web when we are speaking about regulatory strategy for medical devices, three major strategies can cause delays in obtaining approval to market in any country. Explore regulatory options to streamline and modernize timely implementation of Contains nonbinding recommendations (version october 6, 2021) 2 Determining a cost/return on investment for. Ad click now to learn more about rca's global consulting network and service solutions.Regulatory Globe Gap Analysis Template Medical Devices, HD Png
Regulatory Strategies for Medical Device Companies to Succeed in Asia
Is Regulatory compliance strategy for medical devices effective
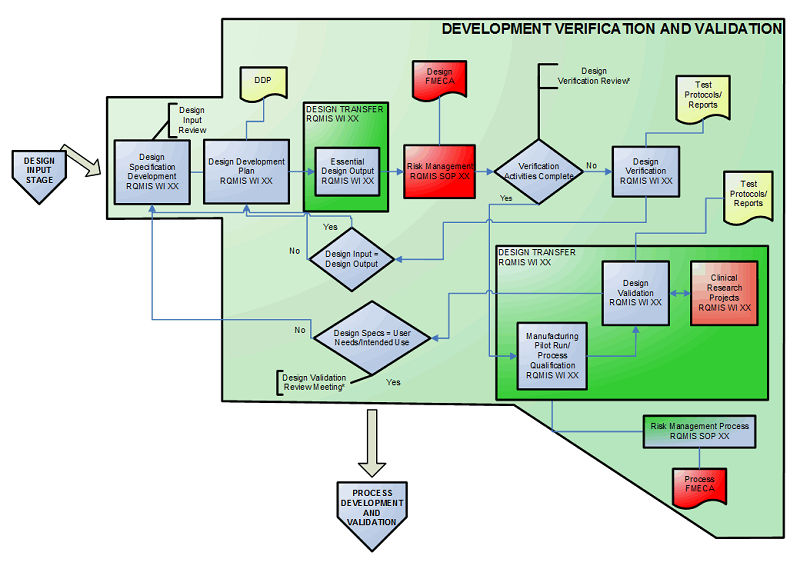
Image result for design control phases medical device Medical device
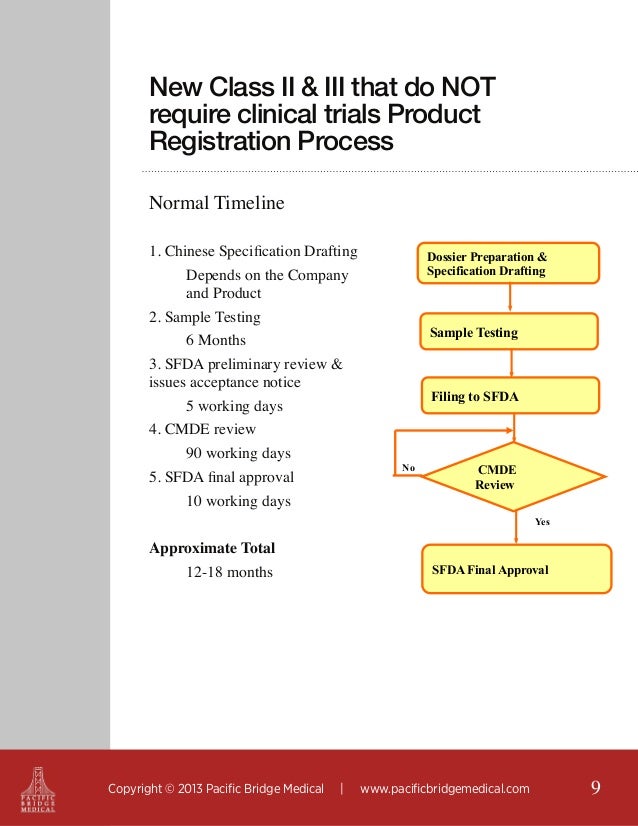
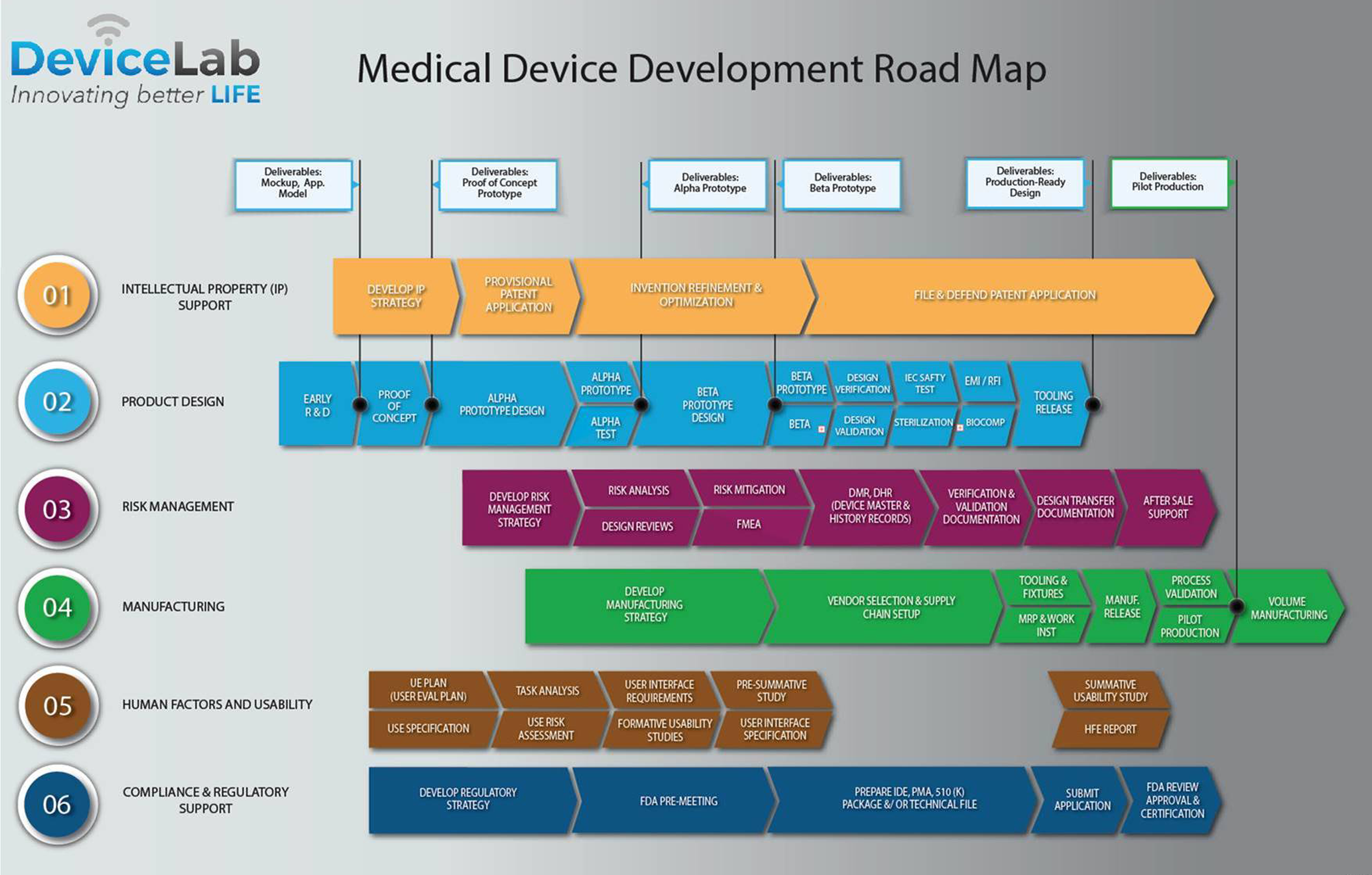
The Medical Device Development Process at DeviceLab Part 1
Medical Devices; US and Chinese legislation Kvalito
Stringent Regulatory Authority The Regulation Of Wearable Medical
Regulatory Compliance printable pdf download
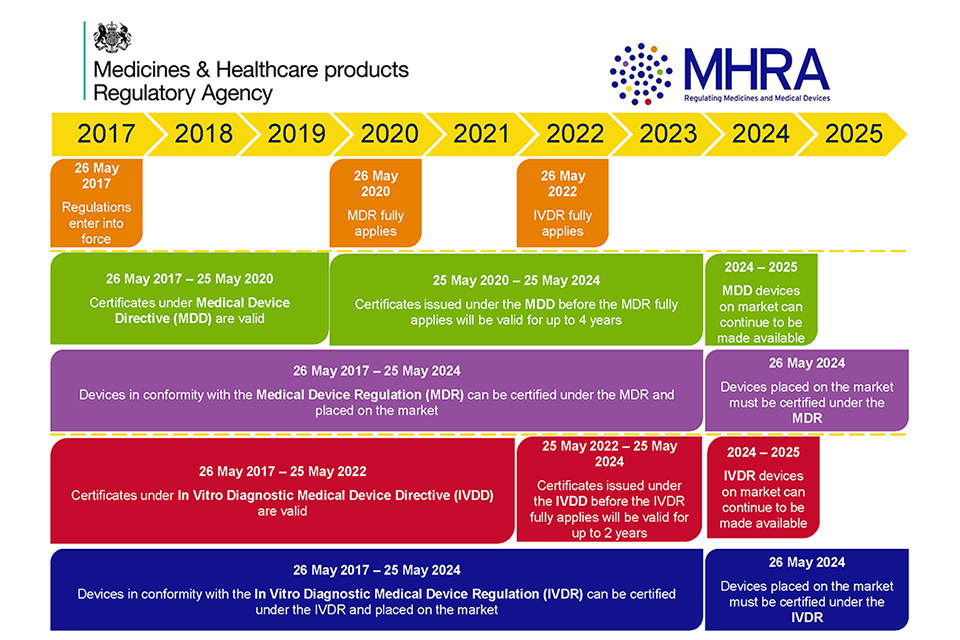
UK MHRA Guidance Medical devices EU regulations for MDR and IVDR
Fda Design Control Guidance Document
Our Strategic Assessments Of Regulatory Requirements Address Business Needs For Medical Device, In Vitro Diagnostic And Combination Product.
Web Here Are The Guidelines:
Web Planning Your Medical Device Global Market Regulatory Strategy.
Frameworks, Tools & Templates To Improve Your Strategic Planning Capability.
Related Post: