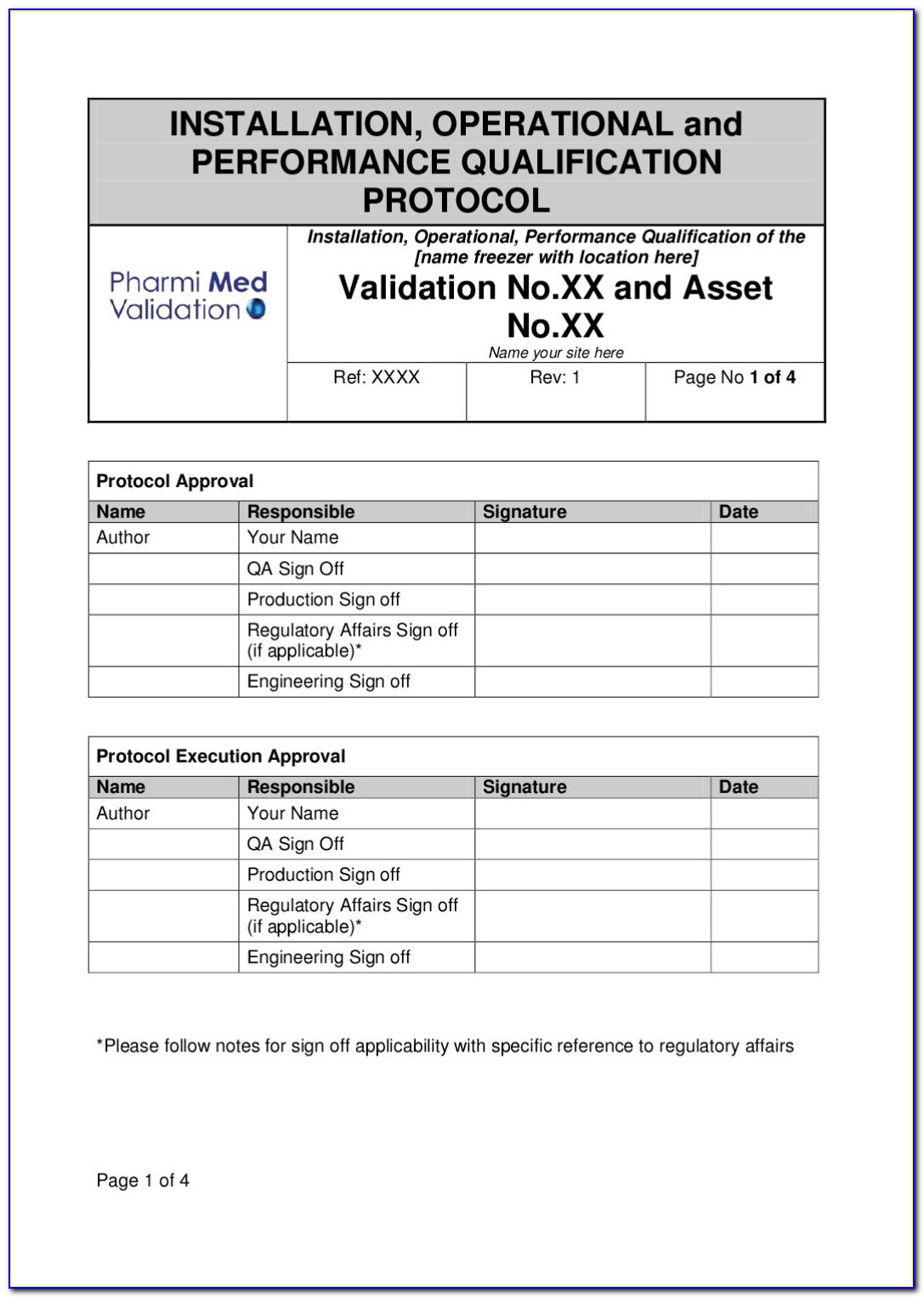
Software Validation Protocol Template
Software Validation Protocol Template - Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software. Trusted by leading pharma, biotech, and medical device companies globally. Web software validation protocol (validation plan): Web long answer validation of computer software is specified in section 4.1.6 of iso 13485:2016. Basically, software verification activities consist of: On completion of each validation batch, a qualification report will be prepared. Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables. Web software validation usually uses three specific testing protocols: Web learn thing can a software validation according for the fda, 21 cfr part 11 and gamp5. Web standardize validation procedures to maintain consistency and efficiency. Web learn thing can a software validation according for the fda, 21 cfr part 11 and gamp5. On completion of each validation batch, a qualification report will be prepared. Ad digitize and manage any validation, commissioning or qualification process. Validate software which is used in. By see tips & download templates, follow us Web software validation protocol (validation plan): Web verification and validation plan template. Web could be safety standard, regulatory standard, customer standards, or company standards. General principles of software validation quote: Validate software which is used in. Web same approval signatories as in the validation protocol & validation report. Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. Trusted by leading pharma, biotech, and medical device companies globally. Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk. Installation qualifications (iq) verify that systems are on machines suited to run the software, that the system has. Web the complete chain of regulatory required documentation for the software validation template of a computer system; Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables. General principles of software validation quote: A document that outlines the. Web could be safety standard, regulatory standard, customer standards, or company standards. A document that outlines the project deliverables and responsibilities. Basically, software verification activities consist of: Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. Web learn thing can a. Web could be safety standard, regulatory standard, customer standards, or company standards. Validate software which is used in. On completion of each validation batch, a qualification report will be prepared. Ad digitize and manage any validation, commissioning or qualification process. Basically, software verification activities consist of: This template is suitable for authoring the tests of either user. Basically, software verification activities consist of: Web verification and validation plan template. General principles of software validation quote: Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. Track and document validation activities to meet regulatory requirements. Web long answer validation of computer software is specified in section 4.1.6 of iso 13485:2016. On completion of each validation batch, a qualification report will be prepared. The main messages there are: Web this software verification and validation procedure provides the action steps for the tank waste information network system (twins). Validate software which is used in. Web the complete chain of regulatory required documentation for the software validation template of a computer system; Installation qualifications (iq) verify that systems are on machines suited to run the software, that the system has. Web verification and validation plan template. On completion of each validation batch, a qualification report will be prepared. Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables. All software changes shall be validatedbefore approval and issuance. Ad digitize and manage any validation, commissioning or qualification process. Web this software verification and validation procedure provides the action steps for the tank waste information network system (twins) testing process. Track and document validation activities to. Web long answer validation of computer software is specified in section 4.1.6 of iso 13485:2016. Installation qualifications (iq) verify that systems are on machines suited to run the software, that the system has. Web standardize validation procedures to maintain consistency and efficiency. Web fda software validation template software validation with the chemical, manufacturing and cannabis enterprises what is browse validation? Web verification and validation plan template. By see tips & download templates, follow us Web the complete chain of regulatory required documentation for the software validation template of a computer system; Trusted by leading pharma, biotech, and medical device companies globally. Web this software verification and validation procedure provides the action steps for the tank waste information network system (twins) testing process. Ad digitize and manage any validation, commissioning or qualification process. Web same approval signatories as in the validation protocol & validation report. Web software validation protocol (validation plan): Web could be safety standard, regulatory standard, customer standards, or company standards. This template is suitable for authoring the tests of either user. Web page 2 guidance for industry and fda staff general principles of software validation in that case, the party with regulatory responsibility (i.e., the device manufacturer) needs to. All software changes shall be validatedbefore approval and issuance. Basically, software verification activities consist of: General principles of software validation quote: Web learn thing can a software validation according for the fda, 21 cfr part 11 and gamp5. Web in house written software (excel/access) for eqr, sop and instrument data capture where validation is based on the risk and nature of the software. Installation qualifications (iq) verify that systems are on machines suited to run the software, that the system has. Web this software verification and validation procedure provides the action steps for the tank waste information network system (twins) testing process. Web standardize validation procedures to maintain consistency and efficiency. Web operational qualifications (oq) and performance qualifications (pq) test protocols are key validation deliverables. All software changes shall be validatedbefore approval and issuance. Validate software which is used in. On completion of each validation batch, a qualification report will be prepared. A document that outlines the project deliverables and responsibilities. Ad digitize and manage any validation, commissioning or qualification process. Track and document validation activities to meet regulatory requirements. This template is suitable for authoring the tests of either user. Web could be safety standard, regulatory standard, customer standards, or company standards. The main messages there are: Web verification and validation plan template. Use this verification and validation plan template to review, inspect, test, audit, and establish whether items, processes, services. By see tips & download templates, follow us10+ Validation Report Templates Free Sample, Example Format Download
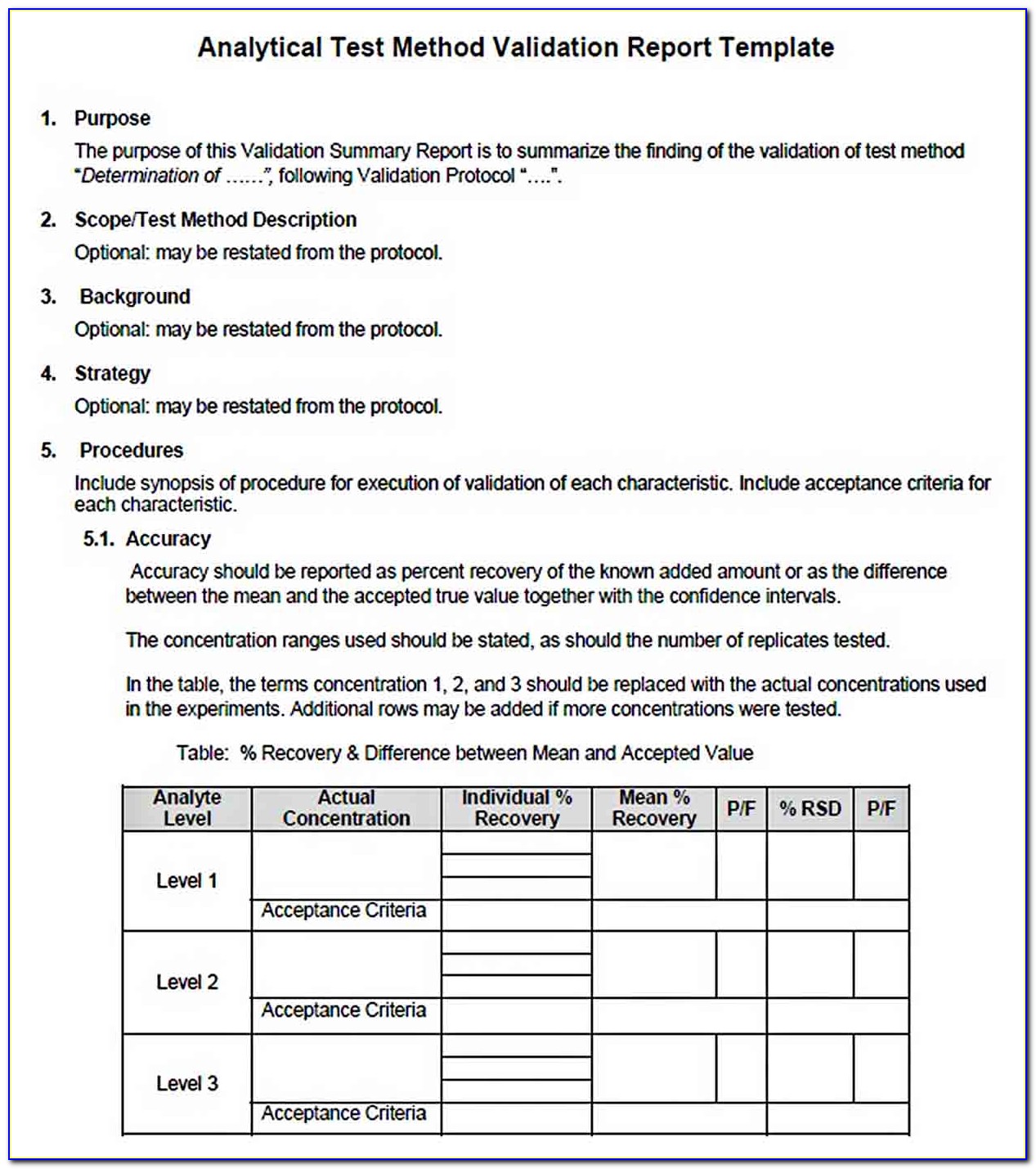
Analytical Method Validation Protocol Sample
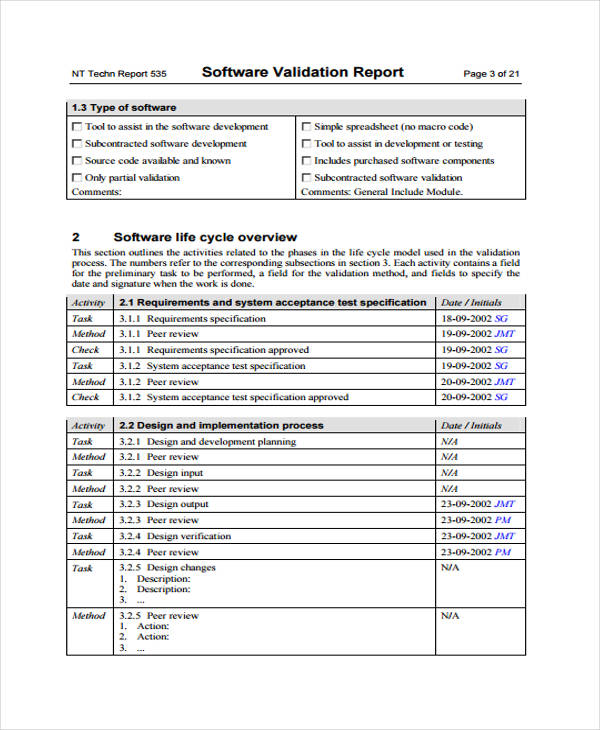
Software Validation Procedure Template
Software Validation Template
Software Validation Protocol Sample
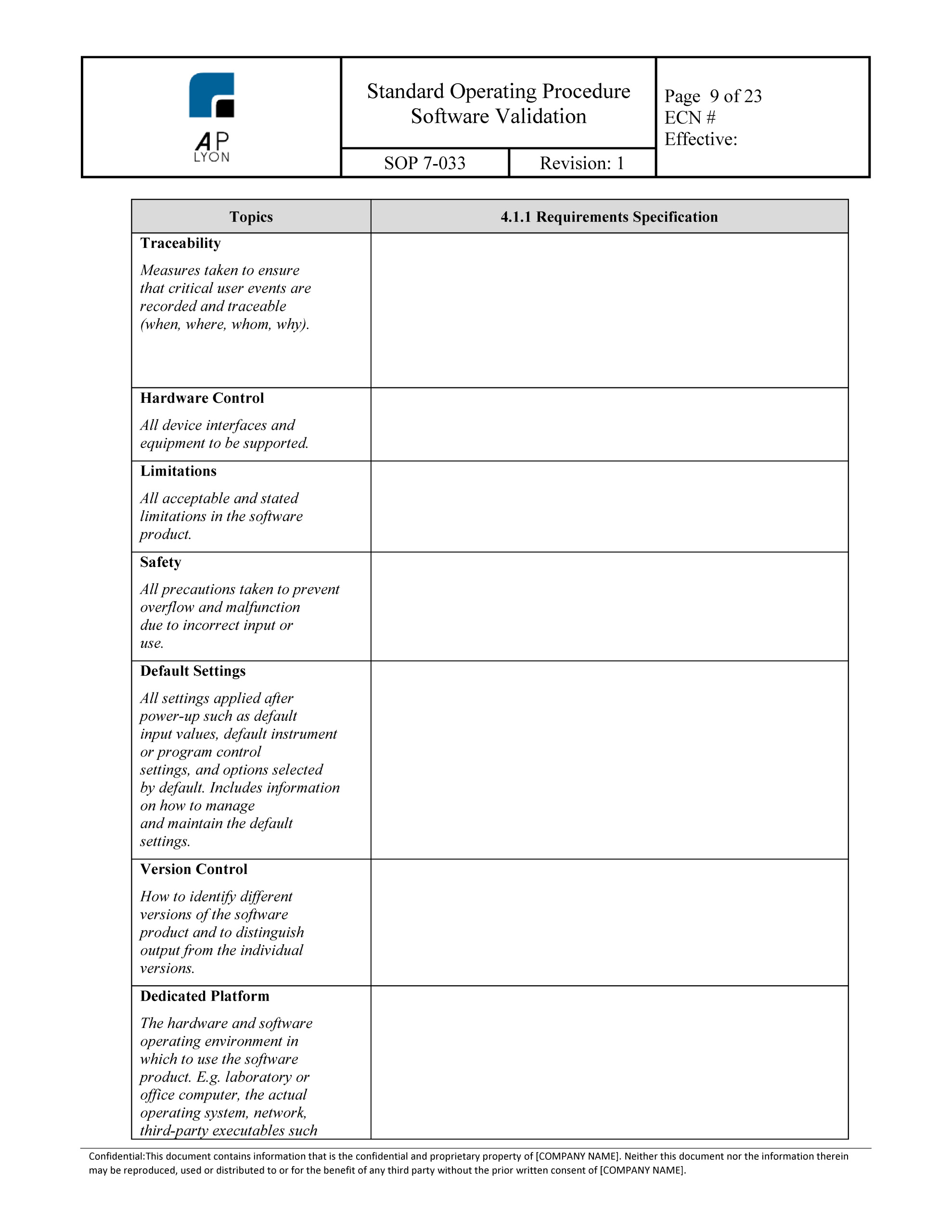
SOFTWARE VALIDATION SOP Template MD48 GMP, QSR & ISO Compliance
Software Validation Templates
SOFTWARE VALIDATION SOP Template PH48 GMP, QSR & ISO Compliance
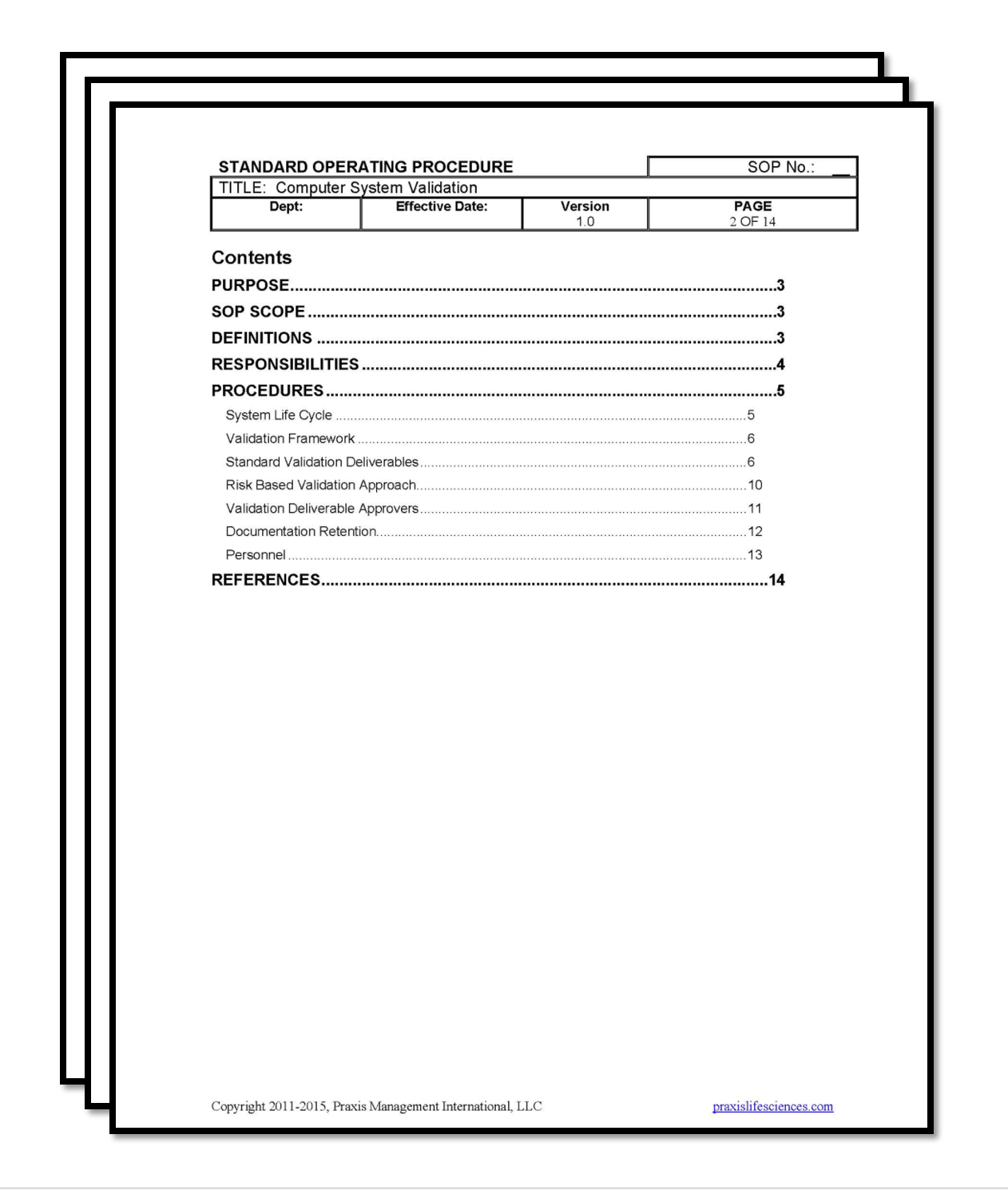
Computer System Validation SOP Validation Center
Iq Oq Pq Software Validation Templates
Trusted By Leading Pharma, Biotech, And Medical Device Companies Globally.
General Principles Of Software Validation Quote:
Web The Complete Chain Of Regulatory Required Documentation For The Software Validation Template Of A Computer System;
Web Long Answer Validation Of Computer Software Is Specified In Section 4.1.6 Of Iso 13485:2016.
Related Post: