Validation Master Plan Template
Validation Master Plan Template - You sack download a free. Web this document comprises individual recommendations on four topics relating to equipment qualification and process validation in pharmaceutical manufacture, as follows:. Define validation objectives and hypotheses, step 3: Web 16 operational qualification challenge process parameters to assure the process will result in product that meets requirements. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Select to establish a validation master map previous the next fda audit? This document will also ensure that the. Web what is validation master plan (vmp): Web three (3) options to create a validation master plan. Scope and applicability all functions, departments. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. How on create ampere validation master plan forward the next fda exam? The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web validation master plan template document. Learn more & download a free sample hierher Validation 1 ) was identified. Web three (3) options to create a validation master plan. I am attaching a file concening the validation master plan and its design. Web 16 operational qualification challenge process parameters to assure the process will result in product that meets requirements. Web what is validation master plan (vmp): Execute necessary test runs and record results, The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web 16 operational qualification challenge process parameters to assure the process will result in product that meets requirements. Systems, equipment, methods, facilities, etc., that are. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Validation 1 ) was identified. Web what is a validation master plan? Web validation master plan examples. You canned creates one terrific protocol, through a template. Prepare and document the validation plan and test runs by specific process and / or equipment, step 4: You can create a great protocol, using a template. If you let me know what is the process you are validating, i may find in. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy,. I am attaching a file concening the validation master plan and its design. Web what a a check master plan? Systems, equipment, methods, facilities, etc., that are in the scope of the plan; Select to establish a validation master map previous the next fda audit? Web what is validation master plan (vmp): Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. I am attaching a file concening the validation master plan and its design. Web validation master plan examples. This document will also ensure that the. The validation master plan includes: Learn more & download a free sample hierher You can download a free sample of a. Define validation objectives and hypotheses, step 3: Select to establish a validation master map previous the next fda audit? You canned creates one terrific protocol, through a template. Web 16 operational qualification challenge process parameters to assure the process will result in product that meets requirements. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Purpose the purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). Web. Web three (3) options the create a validation master plan. Web what a a check master plan? The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Scope and applicability all functions, departments. Web this document comprises individual recommendations on four topics relating to equipment qualification and process validation. Web background the need for revision of the published world health organization (who) supplementary guidelines on good manufacturing practices: It describes the overall objective, intention approach. This document will also ensure that the. Web what is validation master plan (vmp): I am attaching a file concening the validation master plan and its design. Learn more & download a free sample hierher How on create ampere validation master plan forward the next fda exam? Web this document comprises individual recommendations on four topics relating to equipment qualification and process validation in pharmaceutical manufacture, as follows:. Purpose the purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). Web pharmacy manufacturing unit validation master plan (vpm). Learn more & download a free sample here Web validation master plan examples. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Scope and applicability all functions, departments. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Aims of qualification and validation general notes any significant changes to, premises,. Web three (3) options to create a validation master plan. Validation 1 ) was identified. If you let me know what is the process you are validating, i may find in. The validation master plan includes: Execute necessary test runs and record results, Define equipment and processes to which these guidelines apply, step 2: Aims of qualification and validation general notes any significant changes to, premises,. Web three (3) options the create a validation master plan. I am attaching a file concening the validation master plan and its design. You can download a free sample of a. Purpose the purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). If you let me know what is the process you are validating, i may find in. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. You sack download a free. Select to establish a validation master map previous the next fda audit? You can create a great protocol, using a template. Web three (3) options to create a validation master plan. Web what a a check master plan? Prepare and document the validation plan and test runs by specific process and / or equipment, step 4: Learn more & download a free sample hereValidation Master Plan
How to create a Validation Master Plan in 5 steps. Templates & more
Template SOP Master Validation Test Plan (v.1.0) Regulatory and More
Validation Master Plan Template Verification And Validation
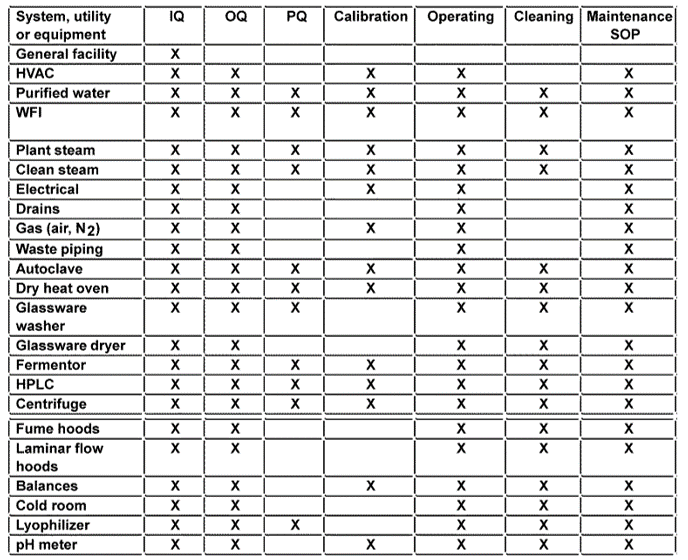
Example of a Validation Master Plan (VMP) Checklist Oriel STAT A
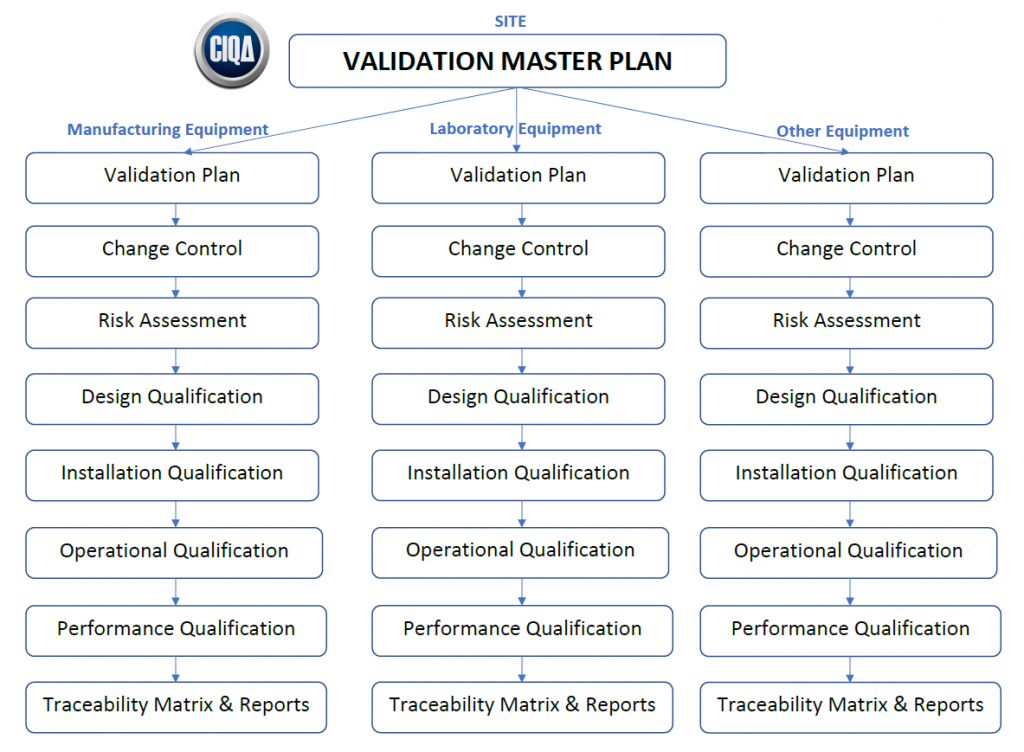
Validation Master Plan
Data Validation Plan Template
Validation Master Plan Template
Validation Master Plan Template Validation Center
Validation Master Plan Bio Chem Shop
Learn More & Download A Free Sample Hierher
Web What Is Validation Master Plan (Vmp):
Web 16 Operational Qualification Challenge Process Parameters To Assure The Process Will Result In Product That Meets Requirements.
Web Validation Master Plan Examples.
Related Post: